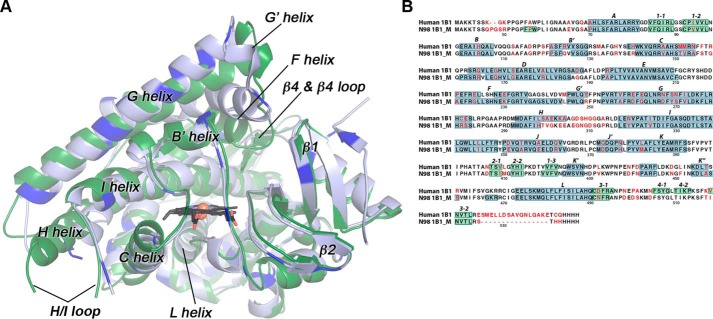
Figure 1.
Comparisons of the mammalian ancestral and human CYP1B1 cytochrome P450 enzymes. A, the global structure of the extant human CYP1B1 (green) compared with that of the ancestral CYP1B1 (blue). Brighter blue in the ancestral CYP1B1 structure indicates the positions of differences in the amino acid sequences. Both were crystallized with α-naphthoflavone (not shown for clarity). The heme is shown as black sticks with the iron as an orange sphere. B, aligned amino acid sequences for both enzymes are annotated with the nonconserved amino acids (in red text) and secondary structure features (helices highlighted in blue; β strands highlighted in green; named above the sequence). Amino acid numbering corresponds to the full-length human CYP1B1 sequence. N98 1B1_M is the N98_CYP1B1_Mammal sequence. Note that the extant human CYP1B1 structure (PDB: 3PM0) was determined from a version of the protein that has a naturally occurring SNP resulting in an A119S substitution.