Figure 1.
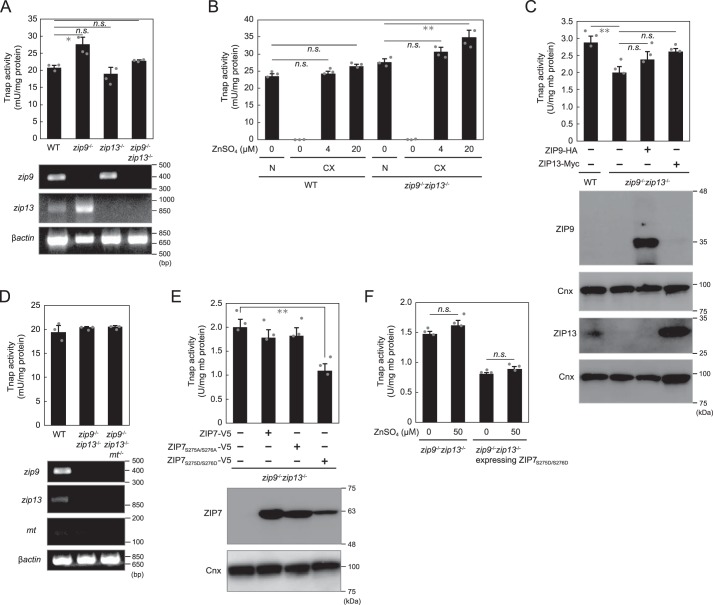
Alteration of expression of ZIPs localized to early secretory pathway does not markedly affect Tnap activity. A, Tnap activity was not substantially altered by the disruption of zip9 or zip13 or both genes (zip9−/−zip13−/−). B, in zinc-deficient cultures (CX), Tnap activity was potently suppressed in zip9−/−zip13−/− cells, similarly as in WT cells. N, normal culture. C, re-expression of ZIP9 or ZIP13 did not notably affect Tnap activity in zip9−/−zip13−/− cells. D, disruption of metallothionein genes (mt) in zip9−/−zip13−/− cells did not alter Tnap activity. E, overexpression of WT ZIP7 or ZIP7 phosphoablative mutant (ZIP7S275A/S276A) did not produce a large effect on Tnap activity, whereas the expression of the ZIP7 phosphomimetic active mutant (ZIP7S275D/S276D) decreased (but did not eliminate) Tnap activity by ∼40%. F, Tnap activity in zip9−/−zip13−/− cells or in zip9−/−zip13−/− cells stably expressing the ZIP7S275D/S276D mutant was not markedly changed by zinc supplementation. A, B, and D, Tnap activity was measured using total cellular proteins. A–F, all activities are expressed as means ± S.D. of triplicate experiments (n = 3). Statistical significance was analyzed by one-way ANOVA followed by Tukey's post hoc test in A–C or by Student's t test in E and F. *, p < 0.05; **, p < 0.01; n.s., not significant. Calnexin (Cnx) was used as the loading control; mb protein, membrane protein. Each experiment was performed at least three times, and representative results from independent experiments are displayed.