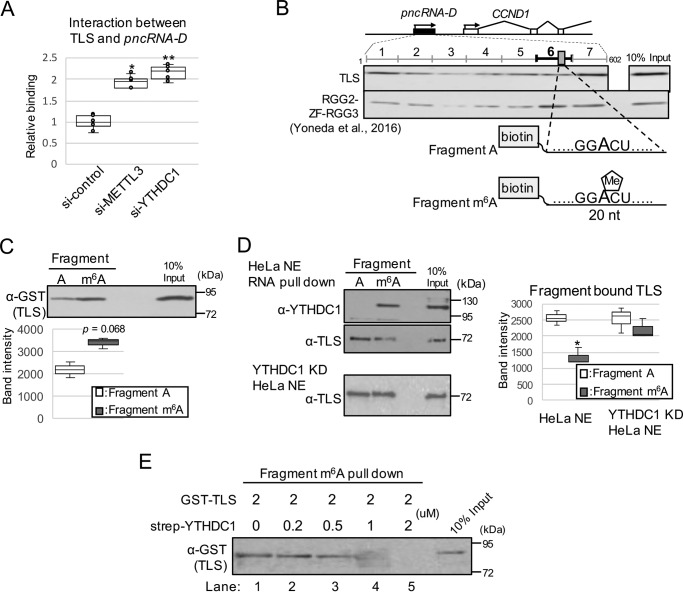
Figure 4.
m6A modification inhibits interaction between pncRNA-D and TLS by competitive binding of YTHDC1. A, RIP assay by TLS antibody with HeLa cells treated with siRNAs of negative control, METTL3, or YTHDC1 (n = 5). *, p < 0.05; **, p < 0.01. B, schematic drawing of full-length pncRNA-D and position of GGACU. The results of Western blotting analysis after RNA pulldown assay were cited from our previous paper in 2016 (9). 20-nt biotinylated fragment around GGACU was generated with (fragment m6A) or without (fragment A) m6A modification. C and D, RNA pulldown assay using fragment A or m6A incubated with GST–TLS (C) or HeLa nuclear extract (D). RNA-bound proteins were detected by the indicated antibodies. Representative images of three individual experiments are shown. The band intensity of TLS bound to fragment A or fragment m6A in HeLa NE or YTHDC1 KD HeLa NE was quantified by ImageJ (RRID:SCR_003070) (D, right) (n = 3); *, p < 0.05. E, RNA pulldown assay using fragment m6A incubated with GST–TLS and various concentration of strep-YTHDC1. Fragment m6A was incubated with GST–TLS and strep-YTHDC1 at the indicated amount, and the RNA bound GST–TLS was detected by Western blotting analysis.