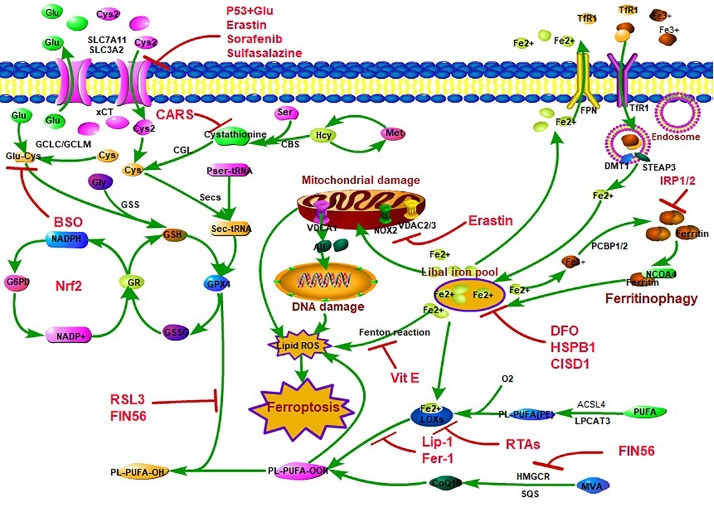
FIGURE 1.
The classic control network of ferroptosis. There are three main characteristics for ferroptosis, including lipid peroxidation, amino acid metabolism disorder, and iron accumulation. The lipid hydroperoxide metabolic pathway is mainly controlled by Acyl-CoA synthetase long-chain family member 4 (ACSL4), lysophosphatidylcholine acyltransferase (LPCAT3), and lipoxygenases (LOXs). ACSL4 catalyzes the attachment of arachidonic acid (AA) or adrenaline (AdA) to produce AA or AdA acyl Co-A derivatives, which is then esterified to phosphatidylethanolamine (PE) (AA-PE and AdA-PE) by lysophosphatidylcholine acyltransferase 3 (LPCAT3). Subsequently, AA-PE and AdA-PE are oxidized by LOXs to produce lipid hydroperoxide, which ultimately leads to ferroptosis. The amino acid metabolism disorder pathway is mainly owing to glutathione (GSH) peroxidase 4 (GPX4) synthesis and function blocking. GPX4 resists iron and oxygen-dependent lipid peroxidation by converting lipid peroxides (L-OOH) to non-toxic lipids and acts as the key enzyme in ferroptosis regulation. GSH is a cofactor and a synthetic substrate for GPX4 and is required for the lipid repair function of GPX4. GPX4 synthesized by GSH requires the pentose–phosphate pathway to supply ATP [through the nicotinamide adenosine dinucleotide hydro-phosphoric acid (NADPH) cycle]. GSH is synthesized by glutamate (Glu), cysteine (Cys), and glycine (Gly) and consists of ATP-dependent glutamate-cysteine ligase (GCL) and GSH synthetase (GSS) through cystine/glutamate reverse transport system xCT [12-channel transmembrane protein transporter vector family 7 member 11 (SLC7A11)/single-channel transmembrane regulatory protein solute carrier family 3 member 2 (SLC3A2)] or sulfur transfer pathway [methionine (Met)–homocysteine (Hcy)–cysteine (Cys) pathway]. When xCT/sulfur transfer pathway is inhibited, the synthesis of GSH and Cys decreasing, which leads to the inhibition of GPX4 synthesis and function to clear LOOH suppression, eventually leading to lipid peroxidation and inducing ferroptosis. The iron accumulation mainly caused by the loss of control of iron transport [membrane iron transporter (FPN), transfer iron protein receptor 1 (TfR1), divalent metal ion transporter 1 (DMT1)] and iron storage [ferritin, degradation via the nuclear receptor coactivator 4 (NCOA4)-mediated ferritinophagy pathway], leading to an increase in the concentration of iron in the labile iron pool (LIP) and an increase in reactive oxygen species (ROS) through Fenton reaction/mitochondrial damage/LOX function.