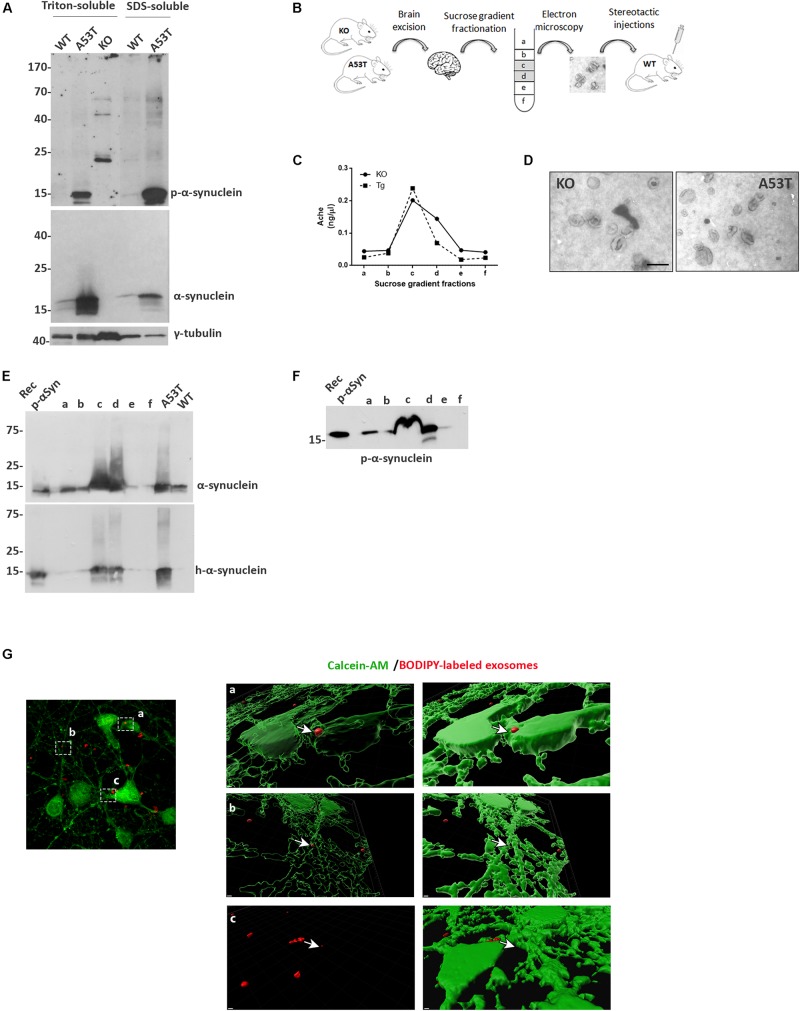
FIGURE 1.
Isolation and characterization of mouse brain exosomes. (A) Brain tissue from wild-type (wt), A53T, and KO mice was separated into Triton X- and sodium dodecyl sulfate (SDS)-soluble fractions. α-Synuclein species were detected by western blot analysis with the Syn-1 and pS129 antibodies. Increased detergent-soluble and detergent-insoluble α-synuclein species were detected in the A53T mice. γ-Tubulin was used as loading control. (B) Schematic representation of the procedure followed for the isolation of whole brain exosomes. (C) Graph depicting the concentration of AchE enzyme contained in the isolated exosomal fractions. (D) Electron microscopy images of isolated exosomes from KO and A53T mouse brains. Scale bar, 200 nm. Human and total (E) as well as phospho-α-synuclein (F) species were detected in the isolated exosomal fractions from A53T mouse brains using the 4B12, Syn-1, and pS129 antibodies, respectively. Recombinant α-syn protein, A53T, and wt brain lysates were used as controls. (G) Primary mouse cortical cultures were incubated in the presence of BODIPY-labeled exosomes (red) for 3 h, and incorporation of the fluorescent lipophilic dye was monitored by confocal microscopy. Neurons were stained with calcein-AM. Internalization of dyed exosomes was assessed by using the Imaris software. Scale bar represents 2 μm.