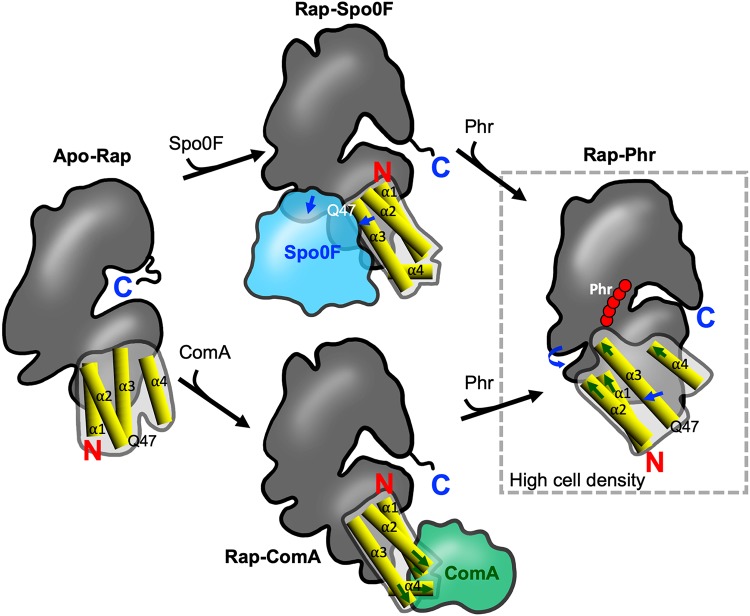
FIG 2.
Schematic representation of the distinct conformations adopted by Rap proteins to switch functions. Shown are Apo-Rap (left), Rap-Spo0F (top), Rap-ComA (bottom), and Rap-Phr (right). The C-terminal TPR domain is shown as a dark-gray outline. The N-terminal domain, composed of α-helixes 1, 2, and 3 (3-helix bundle), and the linker region (α4) are shown as a light-gray outline, and each helix is detailed as yellow cylinders. Sites in the ComA-binding surface of Rap are indicated with green arrows; sites in the Spo0F-binding surface are indicated with blue arrows. Q47 indicates the position of the glutamine catalytic residue for phosphatase activity. C, carboxyl terminus; N, amino terminus. This schematic representation is based on the structures of Rap conformations from reference 44.