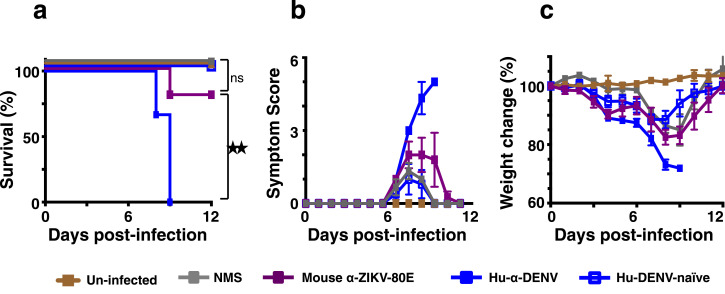
Fig. 8.
Evaluation of ZIKV infection-enhancement by anti-ZIKV-80E NP antiserum. C57BL/6 Stat2−/− mice were inoculated i.p. with 20 µl dengue-positive human plasma (Hu-α-DENV, solid blue squares), 20 µl dengue-negative human plasma (Hu-DENV-naïve, empty blue squares), 20 µl naïve mouse serum (NMS, solid grey squares) or 20 µl anti-ZIKV-80E NP antiserum (mouse α-ZIKV-80E, solid purple squares) and then challenged with live ZIKV (PRVABC59, 5 × 103 PFU/mouse, i.d.) two hours later. One group of mice which received neither immune serum nor ZIKV inoculation (Un-infected, solid brown squares) was included for comparison. Mice were monitored for survival (panel ‘a’), clinical symptoms (scored as described [24], panel ‘b’) and weight loss (panel ‘c’) for a period of 12 days following virus challenge. Differences between the weights of mice in the ‘Hu-α-DENV’, ‘Hu-DENV-naïve’ and ‘mouse α-ZIKV-80E’ groups, for days 3, 4, 5, and 6, analysed using a one way ANOVA (Tukey's test for multiple comparisons), were statistically not significant (p>0.05). Survival in the ‘mouse α-ZIKV-80E’ group was significantly higher than that in ‘Hu-α-DENV of mice’ group (p = 0.027) and comparable to ‘NMS’ group (p = 0.371) and ‘Un-infected’ group (p = 0.438), based on Mantel–Cox test. Statistical differences are indicated as not significant (ns) or as significant (twin star symbol).