Abstract
Objective
To assess the prevalence of diabetes and its risk factors.
Design
Population based, cross sectional study.
Setting
31 provinces in mainland China with nationally representative cross sectional data from 2015 to 2017.
Participants
75 880 participants aged 18 and older—a nationally representative sample of the mainland Chinese population.
Main outcome measures
Prevalence of diabetes among adults living in China, and the prevalence by sex, regions, and ethnic groups, estimated by the 2018 American Diabetes Association (ADA) and the World Health Organization diagnostic criteria. Demographic characteristics, lifestyle, and history of disease were recorded by participants on a questionnaire. Anthropometric and clinical assessments were made of serum concentrations of fasting plasma glucose (one measurement), two hour plasma glucose, and glycated haemoglobin (HbA1c).
Results
The weighted prevalence of total diabetes (n=9772), self-reported diabetes (n=4464), newly diagnosed diabetes (n=5308), and prediabetes (n=27 230) diagnosed by the ADA criteria were 12.8% (95% confidence interval 12.0% to 13.6%), 6.0% (5.4% to 6.7%), 6.8% (6.1% to 7.4%), and 35.2% (33.5% to 37.0%), respectively, among adults living in China. The weighted prevalence of total diabetes was higher among adults aged 50 and older and among men. The prevalence of total diabetes in 31 provinces ranged from 6.2% in Guizhou to 19.9% in Inner Mongolia. Han ethnicity had the highest prevalence of diabetes (12.8%) and Hui ethnicity had the lowest (6.3%) among five investigated ethnicities. The weighted prevalence of total diabetes (n=8385) using the WHO criteria was 11.2% (95% confidence interval 10.5% to 11.9%).
Conclusion
The prevalence of diabetes has increased slightly from 2007 to 2017 among adults living in China. The findings indicate that diabetes is an important public health problem in China.
Introduction
Diabetes is a metabolic disorder caused by genetic and environmental factors, which results in insulin insensitivity, insulin deficiency, and impaired biological function. The disease has become a critical health concern worldwide owing to its high prevalence and related disability and mortality.1 2 The prevalence and number of adults with diabetes has increased at a greater rate in low and middle income countries than in high income countries. This rise in prevalence has been compounded by a growing and ageing population, nearly quadrupling the number of adults with diabetes from 1980 to 2014 worldwide.3 The increase in prevalence could also be partially attributable to the new diagnostic criteria, with reduced cut-off points for blood glucose and the addition of glycated haemoglobin (HbA1c). The American Diabetes Association (ADA) criteria and World Health Organization (WHO) criteria are shown in table 1. The main difference between them is the inclusion of HbA1c in the ADA criteria.4 5
Table 1.
Diagnostic criteria for diabetes related disorders
| Disorders | ADA diagnostic criteria | WHO diagnostic criteria |
|---|---|---|
| Self-reported diabetes | A self-reported diagnosis that was determined previously by a healthcare professional | A self-reported diagnosis that was determined previously by a healthcare professional |
| Newly diagnosed diabetes | Among participants without self-reported diabetes: fasting plasma glucose ≥126 mg/dL (7.0 mmol/L), or oral glucose tolerance test: two hour plasma glucose ≥200 mg/dL (11.1 mmol/L), or HbA1c ≥6.5% | Among participants without self-reported diabetes: fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or oral glucose tolerance test: two hour plasma glucose ≥200 mg/dL (11.1 mmol/L) |
| Total diabetes | Sum of the number of patients with self-reported diabetes and the number of patients with newly diagnosed diabetes | Sum of the number of patients with self-reported diabetes and the number of patients with newly diagnosed diabetes |
| Impaired fasting glucose | Among participants without diabetes: fasting plasma glucose 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L) | Among participants without diabetes: fasting plasma glucose 110 mg/dL (6.1 mmol/L) to 125 mg/dL (6.9 mmol/L), and oral glucose tolerance test: two hour plasma glucose <140 mg/dL (7.8 mmol/L) |
| Impaired glucose tolerance | Among participants without diabetes: oral glucose tolerance test: two hour plasma glucose 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) | Among participants without diabetes: fasting plasma glucose <126 mg/dL (7.0 mmol/L), and oral glucose tolerance test: two hour plasma glucose 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) |
| Prediabetes | Among participants without diabetes: fasting plasma glucose 100 mg/dL (5.6 mmol/L) to 125 mg/dL (6.9 mmol/L), or oral glucose tolerance test: two hour plasma glucose 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L), or HbA1c 5.7-6.4% | Among participants without diabetes: fasting plasma glucose 110 mg/dL (6.1 mmol/L) to 125 mg/dL (6.9 mmol/L), and oral glucose tolerance test: two hour plasma glucose <140 mg/dL (7.8 mmol/L), or fasting plasma glucose <126 mg/dL (7.0 mmol/L), and oral glucose tolerance test: two hour plasma glucose 140 mg/dL (7.8 mmol/L) to 199 mg/dL (11.0 mmol/L) |
ADA=American Diabetes Association; HbA1c=glycated haemoglobin; WHO=World Health Organization.
Rapid economic development in the past three decades has led to a change in lifestyle among the Chinese population, with more sedentary behaviour and a high energy/high fat diet.6 This change has resulted in more people who are overweight.7 The prevalence of diabetes in adults living in China increased from 0.67% using the WHO criteria in 1980 to 10.9% using the ADA criteria in 2013.8 9 10 11 12 13 14 Although different sampling methods and screening procedures were used between 1980 and 2013, these data show a remarkable increase. According to the diabetes atlas of the International Diabetes Federation, China has the largest number of patients with diabetes in the world. Estimates suggest that there are 113.9 million adults with diabetes living in China, accounting for 24% globally of patients with diabetes.15 Additionally, the healthcare cost associated with diabetes in China is 110 billion international dollars (purchasing power parity) in 2017.15
China implemented healthcare reforms in 2013.16 As a result, patients with non-communicable diseases, including diabetes, could have easier access to clinical resources, thus improving the control of diabetes. Some areas have carried out a whole population project with prevention, screening, diagnosis, and management of diabetes and other non-communicable diseases. Prevention and treatment of diabetes has moved from large general hospitals to community health service centres, from simple clinical treatment to tertiary prevention of diabetes, and from simple control of blood glucose to control of weight, blood glucose, blood pressure, and blood lipids.
Our objective was to perform a cross sectional study of the prevalence of diabetes and prediabetes in mainland China, and to evaluate awareness, treatment, and control of diabetes by geographical location and subpopulation.
Methods
Sampling and study population
This epidemiological study, the Thyroid disorders, Iodine status and Diabetes Epidemiological survey (TIDE study), included all 31 provinces of mainland China with a sample of 75 880 people according to the age and sex composition of each community and the urban-rural ratio using the latest national census data.17 The study was conducted between 2015 and 2017.
We used a multistage, stratified sampling method to select a nationally representative sample of people aged 18 and older in the general population. The study was conducted through four stages of random sampling in urban and rural locations in parallel (supplementary fig 1). Developed, developing, and underdeveloped cities were defined based on gross domestic product per capita, concentration of commercial resources, the extent to which a city serves as a commercial hub, vitality of residents, diversity of lifestyle, and future growth potential. Six tiers of cities were categorised from developed to underdeveloped cities, each two tiers being defined as a rank and thus three ranks in all. The cities with tier 1 and new tier 1 were classified as developed cities, tiers 2 and 3 as developing cities, and tiers 4 and tier 5 as underdeveloped cities (supplementary table 1).
For urban locations, at the first stage, one city was selected from each province from all 31 provinces of China. These 31 cities were classified as 10 developed, 13 developing, and 8 underdeveloped cities. At the second stage, one district was randomly selected from each city. At the third stage, two residential communities were randomly selected from each district. At the final stage, eligible people who met the inclusion criteria and were registered as local residents were randomly selected and stratified by age and sex. The composition of age and sex of each community and urban-rural ratio were decided based on China’s 2010 national census data.17 Parallel random sampling was performed in rural locations (supplementary fig 1).
For adult respondents, the inclusion criteria were age 18 or older, living in the selected community for at least five years, and not pregnant. At least 1000 people from each ethnic minority were recruited in four autonomous regions. Finally, Tibetan, Uyghur, Hui, and Zhuang ethnic groups were analysed. A total of 80 937 participants completed the survey and the overall response rate was 92.1%. Our analysis included 75 880 participants after excluding 5057 people with missing information on sex, age, plasma glucose, or HbA1c (supplementary fig 1). Research protocols were approved by the medical ethics committee of China Medical University. All participants provided written informed consent after receiving a thorough explanation of the research procedures.
Demographic and behavioural assessment
For each participant, a trained interviewer used a detailed questionnaire to collect information about demographic variables, behavioural factors, family history of chronic diseases, and personal medical history. Current smoking was defined as having smoked at least 100 cigarettes in one’s life and currently smoking cigarettes. Occasional smokers were defined as currently smoking fewer than 20 cigarettes a day. Regular smokers were defined as currently smoking more than 20 cigarettes a day.
Anthropometric and clinical assessment
Body weight, height, waist circumference, and blood pressure were measured by trained health workers according to standard protocols. Body mass index was calculated by dividing body weight in kg by the square of height in metres. Blood pressure was measured by an electronic blood pressure monitor (Omron HEM-7430, Omron Corporation) on the non-dominant arm twice consecutively with a 10 minute interval between measurements and with the participant in a seated position after five minutes of rest. We defined central obesity as a waist circumference of 90 cm or greater for men and 80 cm or greater for women. We defined generalised overweight as a body mass index of 25 to less than 30, and defined obesity as a body mass index of 30 or higher for both men and women.18 Detailed information for the anthropometric and clinical methods and quality control is given in supplementary appendix 1.
Biochemical assessment
Blood samples were collected from all participants after an overnight fast of at least 10 hours. Serum samples were used for the measurements of fasting plasma glucose levels and two hour plasma glucose levels after carrying out an oral 75 g glucose tolerance test. HbA1c was measured in venous blood samples by high performance liquid chromatography (Bio-Rad VARIANT II Haemoglobin Analyzer). In people with self-reported diabetes, only fasting plasma glucose and HbA1c were measured. Fasting plasma glucose, two hour plasma glucose levels, serum total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, and triglycerides were measured using an automatic biochemical analyser (Mindray BS-180 Analyzer).
This biochemical assessment of diabetes differs from the testing strategy used in practice. In the absence of unequivocal hyperglycaemia or symptoms, assessment should be confirmed by repeat testing. We carried out only one reading on measured glucose or HbA1c, but this approach has been widely used by previous national epidemiologic surveys.8 9 10 11 12 13
Outcome assessment
Diagnostic criteria for diabetes related disorders are presented in table 1. Treatment was defined as the proportion of individuals taking drugs for diabetes among those diagnosed with the disease. Control was defined as the proportion of individuals with an HbA1c concentration of less than 7.0% among patients with diabetes who were taking medication.
Statistical analysis
To account for the complex sampling design of this study, we used SUDAAN software (Research Triangle Institute) to obtain estimates of prevalence and the standard errors according to the Taylor linearisation method. Estimates were weighted to reflect age, sex, and urban-rural, distribution of provinces of the adults living in China. Weighting coefficients were derived from the 2010 Chinese population census data, and the sampling scheme of our survey was to obtain a national estimate. Briefly, the weighting coefficient was the inverse of the adjusted probability of obtaining the data for the respondent; each individual case in the analysis was assigned a certain coefficient (individual weight), by which it was multiplied to represent the actual population with the same characteristics of sex, age, province, and location. Standard errors were calculated with appropriate statistical techniques with data from the complex survey design. Categorical data are presented as percentages and 95% confidence intervals and were analysed by a χ2 test or Fisher’s exact test, as appropriate. Continuous data are described with means and 95% CIs. A binomial logit regression was used to examine the association of risk factors with the odds of total diabetes and prediabetes. A P value less than 0.05 was considered statistically significant. All statistical analyses were conducted using the SAS system, version 9.3 (SAS Institute Inc, Cary, NC) and SUDAAN software, version 10.0 (Research Triangle Institute).
Patient and public involvement
This research was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy.
Results
The general characteristics and metabolic risk factors of the study population are presented in table 2 and supplementary figure 2. The overall standardised prevalence of total diabetes, self-reported diabetes, and newly diagnosed diabetes using the ADA criteria were 12.8% (95% confidence interval 12.0% to 13.6%), 6.0% (5.4% to 6.7%), and 6.8% (6.1% to 7.4%), respectively, in Chinese adults. The prevalence of total diabetes was higher in men than in women. The standardised prevalence of prediabetes was 35.2% (33.5% to 37.0%) in Chinese adults, diagnosed by the ADA criteria (table 3 and supplementary table 2). No significant differences in the prevalence of diabetes and prediabetes were found between urban and rural residents. The weighted prevalence of total diabetes diagnosed by the WHO criteria was 11.2% in 2017. The prevalence of diabetes varied with different diagnostic criteria.
Table 2.
General characteristics of adults living in mainland China. Data are percentage (95% confidence interval) unless indicated otherwise
| Characteristics |
Overall (n=75 880) | Sex | Urbanisation | |||
|---|---|---|---|---|---|---|
| Men (n=36 819) | Women (n=39 061) | Urban (n=40 560) | Rural (n=35 320) | |||
| Mean age at survey (95% CI) | 42.8 (41.9 to 43.7) | 42.6 (41.8 to 43.5) | 43.0 (42.1 to 43.9) | 41.4 (40.5 to 42.4) | 44.3 (43.5 to 45.1) | |
| Ethnicity: | ||||||
| Han | 95.4 (93.5 to 96.8) | 95.3 (93.4 to 96.7) | 95.6 (93.6 to 97.0) | 96.7 (86.9 to 99.2) | 94.0 (78.7 to 98.5) | |
| Tibetan | 0.2 (0.1 to 0.5) | 0.2 (0.1 to 0.5) | 0.2 (0.1 to 0.5) | 0.1 (0.01 to 0.7) | 0.3 (0.04 to 2.3) | |
| Uyghur | 1.3 (1.1 to 1.6) | 1.4 (1.1 to 1.7) | 1.3 (1.1 to 1.5) | 1.2 (0.2 to 8.7) | 1.5 (0.2 to 10.9) | |
| Hui | 0.3 (0.1 to 1.3) | 0.3 (0.04 to 1.4) | 0.3 (0.1 to 1.3) | 0.04 (0.01 to 0.3) | 0.5 (0.1 to 3.7) | |
| Zhuang | 2.8 (1.6 to 4.8) | 2.9 (1.7 to 4.8) | 2.7 (1.6 to 4.8) | 2.0 (0.3 to 13.9) | 3.7 (0.5 to 23.7) | |
| Income per year (¥): | ||||||
| ≤30 000 | 44.8 (38.2 to 51.7) | 41.3 (35.1 to 47.9) | 48.4 (41.3 to 55.6) | 31.0 (26.4 to 35.9) | 59.7 (53.9 to 65.3) | |
| >30 000 | 55.2 (48.3 to 61.8) | 58.7 (52.2 to 64.9) | 51.6 (44.4 to 58.7) | 69.1 (64.1 to 73.6) | 40.3 (34.7 to 46.1) | |
| Education: | ||||||
| Less than high school | 43.7 (36.5 to 51.2) | 40.4 (33.6 to 47.6) | 47.1 (39.3 to 55.0) | 28.2 (23.2 to 33.8) | 60.5 (56.2 to 64.7) | |
| High school and above | 56.3 (48.8 to 63.5) | 59.6 (52.4 to 66.4) | 52.9 (45.1 to 60.7) | 71.8 (66.2 to 76.8) | 39.5 (35.3 to 43.8) | |
| Cigarette smoking: | ||||||
| Current non-smoker | 73.6 (72.3 to 74.8) | 50.0 (47.5 to 52.5) | 97.5 (96.8 to 98.1) | 75.5 (73.7 to 77.2) | 71.5 (69.3 to 73.6) | |
| Occasional smoker | 4.0 (3.6 to 4.4) | 7.0 (6.3 to 7.7) | 0.9 (0.7 to 1.2) | 4.3 (3.6 to 5.1) | 3.6 (3.0 to 4.3) | |
| Regular smoker | 22.5 (21.2 to 23.8) | 43.1 (40.4 to 45.8) | 1.6 (1.1 to 2.2) | 20.3 (18.5 to 22.2) | 24.9 (22.5 to 27.4) | |
| Family history of diabetes | 16.4 (13.9 to 19.3) | 15.5 (13.2 to 18.1) | 17.4 (14.6 to 20.6) | 22.0 (19.1 to 25.1) | 10.4 (8.8 to 12.3) | |
| Physical examination: | ||||||
| Mean body mass index (95% CI) | 24.0 (23.9 to 24.1) | 24.6 (24.4 to 24.8) | 23.4 (23.2 to 23.6) | 23.9 (23.6 to 24.3) | 24.1 (23.8 to 24.4) | |
| Body mass index <25 | 62.9 (61.5 to 64.2) | 56.1 (54.2 to 58.0) | 69.7 (67.8 to 71.6) | 63.9 (60.2 to 67.3) | 61.8 (58.5 to 65.0) | |
| Body mass index 25 to <30 | 30.9 (29.8 to 32.0) | 36.3 (34.8 to 37.9) | 25.3 (23.7 to 27.0) | 30.0 (27.6 to 32.6) | 31.8 (29.5 to 34.2) | |
| Body mass index ≥30 | 6.3 (5.9 to 6.6) | 7.6 (7.0 to 8.2) | 4.9 (4.6 to 5.3) | 6.1 (5.1 to 7.4) | 6.4 (5.4 to 7.5) | |
| Mean waist circumference (95% CI; cm) | 83.2 (82.4 to 84.0) | 86.6 (85.8 to 87.5) | 79.7 (78.8 to 80.6) | 82.7 (81.6 to 83.8) | 83.7 (82.1 to 85.3) | |
| Mean heart rate (95% CI; counts per min | 79.8 (79.2 to 80.5) | 78.9 (78.2 to 79.6) | 80.8 (80.1 to 81.5) | 80.0 (78.7 to 81.4) | 79.6 (78.5 to 80.8) | |
| Mean systolic blood pressure (95% CI; mm Hg) | 126.3 (124.9 to 127.7) | 129.9 (128.8 to 131.0) | 122.7 (120.9 to 124.4) | 124.1 (122.8 to 125.5) | 128.7 (126.9 to 130.5) | |
| Mean diastolic blood pressure 95% CI; mm Hg) | 78.3 (77.2 to 79.5) | 80.7 (79.3 to 82.1) | 76.0 (74.9 to 77.0) | 77.3 (76.0 to 78.6) | 79.5 (77.4 to 81.5) | |
| Laboratory tests (mean (95% CI)): | ||||||
| Cholesterol (mmol/L) | 4.8 (4.7 to 4.8) | 4.8 (4.8 to 4.9) | 4.8 (4.7 to 4.8) | 4.8 (4.7 to 4.9) | 4.7 (4.6 to 4.9) | |
| Low density lipoprotein (mmol/L) | 2.8 (2.8 to 2.9) | 2.9 (2.8 to 3.0) | 2.8 (2.7 to 2.8) | 2.8 (2.7 to 2.9) | 2.8 (2.7 to 3.0) | |
| High density lipoprotein (mmol/L) | 1.5 (1.5 to 1.5) | 1.4 (1.4 to 1.4) | 1.6 (1.5 to 1.6) | 1.5 (1.4 to 1.5) | 1.5 (1.4 to 1.5) | |
| Triglycerides (mmol/L) | 1.6 (1.5 to 1.6) | 1.8 (1.7 to 1.8) | 1.4 (1.3 to 1.4) | 1.5 (1.5 to 1.6) | 1.6 (1.5 to 1.7) | |
| Fasting plasma glucose (mmol/L) | 5.4 (5.4 to 5.5) | 5.5 (5.4 to 5.6) | 5.3 (5.3 to 5.4) | 5.4 (5.3 to 5.5) | 5.5 (5.3 to 5.6) | |
| Two hour plasma glucose (mmol/L) | 6.5 (6.4 to 6.6) | 6.5 (6.4 to 6.6) | 6.5 (6.4 to 6.6) | 6.5 (6.4 to 6.7) | 6.5 (6.3 to 6.7) | |
| HbA1c | 5.6 (5.4 to 5.7) | 5.6 (5.5 to 5.7) | 5.5 (5.4 to 5.6) | 5.5 (5.3 to 5.7) | 5.6 (5.6 to 5.7) | |
100=£12; €13; $14.
Table 3.
Age and sex standardised prevalence of diabetes, prediabetes, and risk factors among adults living in mainland China
| Characteristics | No of participants | Percentage estimated prevalence (95% confidence interval) | ||||
|---|---|---|---|---|---|---|
| Self-reported diabetes | Self-reported diabetes or fasting plasma glucose ≥7 mmol/L | Self-reported diabetes, fasting plasma glucose ≥7 mmol/L, or two hour plasma glucose ≥11.1 mmol/L | Self-reported diabetes, fasting plasma glucose ≥7 mmol/L, two hour plasma glucose ≥11.1 mmol/L, or HbA1c ≥6.5% | Prediabetes | ||
| Overall | 75 880 | 6.0 (5.4 to 6.7) | 8.7 (8.1 to 9.3) | 11.2 (10.5 to 11.9) | 12.8 (12.0 to 13.6) | 35.2 (33.5 to 37.0) |
| Sex: | ||||||
| Men | 36 819 | 6.4 (5.6 to 7.2) | 9.7 (8.9 to 10.5) | 12.1 (11.3 to 13.0) | 13.7 (12.8 to 14.7) | 37.0 (35.2 to 38.9) |
| Women | 39 061 | 5.6 (5.1 to 6.2) | 7.7 (7.1 to 8.3) | 10.3 (9.5 to 11.1) | 11.8 (10.9 to 12.7) | 33.4 (31.6 to 35.3) |
| P for difference | — | 0.01 | <0.001 | <0.001 | <0.001 | <0.001 |
| Urbanisation: | ||||||
| Urban | 40 560 | 7.1 (6.6 to 7.7) | 9.6 (9.0 to 10.3) | 12.2 (11.4 to 13.1) | 13.7 (12.7 to 14.7) | 34.6 (31.6 to 37.7) |
| Rural | 35 320 | 5.0 (4.3 to 5.9) | 7.9 (7.0 to 8.9) | 10.3 (9.2 to 11.6) | 12.0 (10.7 to 13.3) | 35.8 (32.2 to 39.6) |
| P for difference | — | 0.08 | 0.49 | 0.76 | 0.92 | 0.29 |
| Age group: | ||||||
| 18-29 | 17 873 | 0.8 (0.5 to 1.2) | 1.3 (0.9 to 1.8) | 1.5 (1.0 to 2.1) | 2.0 (1.5 to 2.7) | 20.2 (18.2 to 22.40) |
| 30-39 | 15 082 | 2.6 (2.1 to 3.2) | 4.2 (3.6 to 4.8) | 5.4 (4.6 to 6.3) | 6.3 (5.4 to 7.3) | 29.9 (27.4 to 32.5) |
| 40-49 | 16 686 | 4.8 (4.1 to 5.7) | 8.2 (7.4 to 9.1) | 10.6 (9.6 to 11.6) | 12.1 (11.1 to 13.3) | 40.0 (38.0 to 41.9) |
| 50-59 | 12 736 | 10.6 (9.6 to 11.7) | 15.0 (14.1 to 16.1) | 18.9 (17.8 to 20.1) | 21.1 (19.8 to 22.6) | 47.1 (44.9 to 49.4) |
| 60-69 | 8205 | 14.9 (12.8 to 17.3) | 19.7 (17.6 to 22.0) | 25.5 (23.3 to 27.9) | 28.8 (26.5 to 31.3) | 47.8 (45.0 to 50.6) |
| ≥70 | 5298 | 16.5 (13.8 to 19.5) | 21.4 (18.6 to 24.4) | 28.8 (25.7 to 32.1) | 31.8 (28.8 to 35.1) | 47.6 (44.3 to 51.0) |
| P for trend | — | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Ethnicity: | ||||||
| Han | 68 064 | 6.1 (5.5 to 6.8) | 8.8 (8.2 to 9.5) | 11.3 (10.6 to 12.0) | 12.8 (12.0 to 13.7) | 35.4 (33.6 to 37.3) |
| Tibetan | 2034 | 1.5 (0.6 to 3.3) | 1.9 (0.7 to 5.0) | 4.2 (3.8 to 4.6) | 6.5 (6.1 to 6.9) | 34.4 (26.3 to 43.6) |
| Uyghur | 2159 | 4.6 (4.2 to 5.0) | 7.8 (6.4 to 9.5) | 9.1 (7.4 to 11.0) | 11.5 (9.6 to 13.6) | 20.2 (12.6 to 30.7) |
| Hui | 1661 | 1.8 (0.5 to 7.0) | 4.4 (2.6 to 7.3) | 5.4 (3.6 to 8.1) | 6.3 (3.9 to 9.9) | 36.2 (31.3 to 41.4) |
| Zhuang | 1962 | 3.8 (1.5 to 9.1) | 5.6 (2.5 to 12.3) | 10.0 (6.6 to 14.7) | 11.4 (7.7 to 16.5) | 35.5 (34.6 to 36.4) |
| P for difference | — | <0.001 | <0.001 | <0.001 | <0.001 | 0.005 |
| Region: | ||||||
| South | 6882 | 5.8 (3.0 to 10.7) | 8.3 (5.3 to 12.7) | 11.6 (8.2 to 16.1) | 12.8 (9.5 to 16.9) | 35.1 (32.7 to 37.1) |
| North | 12 112 | 6.6 (5.2 to 8.4) | 9.2 (7.7 to 10.9) | 11.4 (9.7 to 13.4) | 14.2 (12.5 to 16.2) | 37.4 (33.0 to 42.0) |
| East | 17 206 | 5.9 (5.1 to 6.7) | 8.2 (7.5 to 9.1) | 10.7 (9.9 to 11.5) | 12.2 (10.9 to 13.5) | 33.7 (30.1 to 37.5) |
| Central | 7823 | 6.6 (5.5 to 7.9) | 8.7 (7.8 to 9.6) | 10.5 (9.8 to 11.2) | 12.3 (11.7 to 12.8) | 38.0 (35.4 to 40.7) |
| Southwest | 11 347 | 4.7 (3.7 to 6.1) | 8.8 (7.5 to 10.4) | 11.9 (10.0 to 14.2) | 13.3 (10.8 to 16.3) | 37.7 (31.8 to 44.1) |
| Northwest | 13 147 | 5.7 (4.4 to 7.4) | 8.2 (7.0 to 9.6) | 10.7 (9.6 to 12.0) | 12.1 (10.9 to 13.6) | 31.7 (28.3 to 35.3) |
| Northeast | 7363 | 6.8 (4.8 to 9.6) | 10.0 (7.7 to 12.8) | 12.7 (10.2 to 15.6) | 12.9 (10.5 to 15.8) | 31.3 (24.7 to 38.7) |
| P for difference | — | 0.25 | 0.27 | 0.33 | 0.10 | 0.04 |
| Income per year (¥): | ||||||
| ≤30 000 | 32 339 | 5.9 (5.2 to 6.6) | 8.7 (7.9 to 9.5) | 11.3 (10.4 to 12.3) | 12.9 (11.9 to 14.0) | 35.7 (33.8 to 37.7) |
| >30 000 | 42 552 | 6.3 (5.7 to 7.0) | 8.9 (8.3 to 9.6) | 11.4 (10.7 to 12.1) | 12.9 (12.0 to 13.8) | 35.0 (32.9 to 37.1) |
| P for difference | — | <0.001 | <0.001 | <0.001 | 0.001 | 0.01 |
| Education: | ||||||
| Less than high school | 34 856 | 5.8 (5.1 to 6.7) | 8.8 (7.9 to 9.8) | 11.3 (10.3 to 12.5) | 12.9 (11.8 to 14.1) | 36.9 (34.2 to 39.7) |
| High school and above | 40 729 | 6.6 (6.1 to 7.3) | 9.0 (8.4 to 9.6) | 11.5 (10.8 to 12.2) | 13.2 (12.5 to 14.0) | 34.6 (32.7 to 36.5) |
| P for difference | — | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Cigarette smoking: | ||||||
| Current non-smoker | 55 958 | 6.2 (5.5 to 6.9) | 8.7 (8.1 to 9.4) | 11.4 (10.7 to 12.1) | 12.9 (12.1 to 13.7) | 35.0 (33.2 to 36.9) |
| Occasional smoker | 2626 | 6.2 (4.6 to 8.3) | 9.9 (8.6 to 11.2) | 12.0 (10.9 to 13.3) | 13.5 (12.2 to 14.9) | 35.2 (31.5 to 39.0) |
| Regular smoker | 17 246 | 6.7 (5.7 to 7.9) | 10.0 (8.7 to 11.5) | 11.9 (10.4 to 13.6) | 13.6 (11.9 to 15.4) | 34.2 (31.4 to 37.1) |
| P for trend | — | 0.38 | 0.08 | 0.42 | 0.33 | 0.50 |
| Family history of diabetes: | ||||||
| Yes | 12 348 | 15.3 (14.1 to 16.5) | 18.6 (17.4 to 19.9) | 21.6 (20.2 to 23.2) | 23.3 (21.8 to 25.0) | 33.2 (30.9 to 35.4) |
| No | 63 503 | 4.5 (4.1 to 5.0) | 7.1 (6.6 to 7.6) | 9.5 (8.9 to 10.1) | 11.0 (10.3 to 11.7) | 35.4 (33.6 to 37.2) |
| P for difference | — | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Body mass index: | ||||||
| <25 | 47 749 | 5.0 (4.4 to 5.7) | 6.8 (6.2 to 7.5) | 8.8 (8.0 to 9.6) | 10.0 (9.2 to 10.9) | 33.0 (31.1 to 35.0) |
| 25 to <30 | 23 178 | 7.4 (6.7 to 8.2) | 10.8 (10.0 to 11.7) | 13.8 (13.0 to 14.7) | 15.6 (14.7 to 16.6) | 38.9 (36.6 to 41.4) |
| ≥30 | 4786 | 9.8 (8.8 to 10.9) | 15.3 (14.4 to 16.2) | 20.1 (18.9 to 21.5) | 23.0 (21.8 to 24.2) | 43.1 (40.3 to 46.0) |
| P for trend | — | <0.001 | <0.001 | <0.001 | 0.03 | <0.001 |
| Waist circumference (cm): | ||||||
| Men ≥90, women ≥80 | 41 736 | 7.4 (6.7 to 8.3) | 11.1 (10.3 to 11.9) | 14.2 (13.4 to 15.2) | 16.2 (15.2 to 17.1) | 38.9 (36.9 to 40.9) |
| Men <90, women <80 | 33 827 | 4.4 (3.8 to 5.1) | 6.2 (5.5 to 7.0) | 8.0 (7.2 to 8.9) | 9.3 (8.3 to 10.3) | 33.1 (31.2 to 35.1) |
| P for difference | — | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
100=£12; €13; $14. Some values were missing in the total population: 989 for income, 295 for education, 50 for cigarette smoking, 29 for family history of diabetes, 167 for body mass index, and 317 for waist circumference.
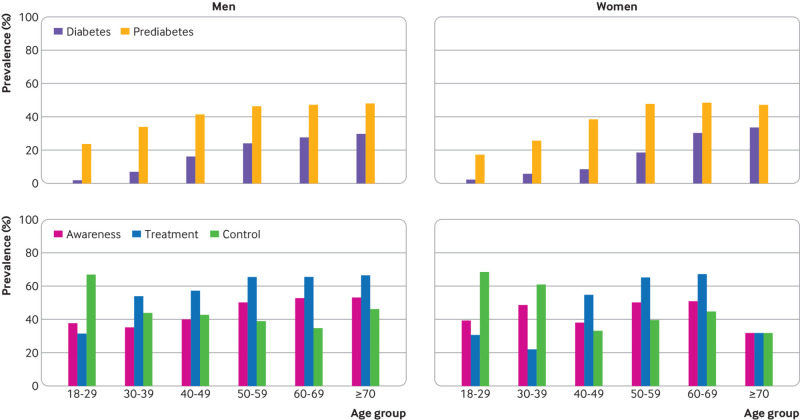
In many subpopulations, the prevalence of total diabetes and prediabetes was higher among men, among people with a low education level and low family income, among those who were overweight and obese, and among those who reported a family history of diabetes. Han ethnic participants had the highest prevalence of total diabetes in comparison with Uyghur, Zhuang, Tibetan, and Hui participants. The prevalence of total diabetes and prediabetes increased with age in both men and women and increased more sharply after age 50 (table 3 and fig 1).
Fig 1.
Prevalence, awareness, treatment, and control of diabetes diagnosed by the American Diabetes Association criteria among study participants
Regional variations in the prevalence of total diabetes were found, with the highest prevalence in the north, followed by the southwest, northeast, south, central, east, and northwest (table 3). Supplementary figure 3 illustrates the geographical variation in the prevalence of total diabetes and prediabetes at the provincial level, stratified into quintiles. The prevalence of total diabetes varied from 6.2% in Guizhou to 19.9% in Inner Mongolia (supplementary table 3).
Awareness, treatment, and control of diabetes and risk factors in adults living in China, standardised for age and sex, are presented in table 4. The proportion of patients who were aware of their diabetes and were treated was higher in the older population, and the rate of awareness of diabetes was significantly higher in urban than in rural residents (table 4 and fig 1). The proportion of patients who controlled their HbA1c levels well was higher in younger patients and in urban residents.
Table 4.
Age and sex standardised awareness, treatment, and control of diabetes and risk factors among adults living in mainland China
| Variables |
Awareness of diabetes | Treatment of diabetes | Control of diabetes | |||||
|---|---|---|---|---|---|---|---|---|
| No of participants | % (95% CI) | No of participants | % (95% CI) | No of participants | % (95% CI) | |||
| Overall | 9772 | 43.3 (39.2 to 47.5) | 4464 | 49.0 (44.5 to 53.4) | 2792 | 49.4 (39.4 to 59.4) | ||
| Sex: | ||||||||
| Men | 5093 | 42.0 (35.6 to 48.7) | 2316 | 52.6 (47.5 to 57.5) | 1503 | 48.0 (37.1 to 59.1) | ||
| Women | 4679 | 44.5 (41.5 to 47.6) | 2148 | 45.3 (38.8 to 51.9) | 1289 | 50.9 (38.8 to 62.8) | ||
| P for difference | — | 0.25 | — | 0.10 | — | 0.36 | ||
| Urbanisation: | ||||||||
| Urban | 5561 | 47.5 (42.9 to 52.0) | 2751 | 51.6 (46.9 to 56.2) | 1746 | 53.9 (41.9 to 65.5) | ||
| Rural | 4211 | 38.0 (33.1 to 43.2) | 1713 | 45.5 (38.6 to 52.7) | 1046 | 47.4 (42.6 to 52.2) | ||
| P for difference | — | 0.02 | — | 0.90 | — | 0.003 | ||
| Age group: | ||||||||
| 18-29 | 344 | 38.4 (28.7 to 49.1) | 118 | 31.0 (20.5 to 43.9) | 38 | 67.7 (32.3 to 90.2) | ||
| 30-39 | 903 | 41.6 (37.0 to 46.5) | 355 | 38.1 (31.9 to 44.7) | 139 | 52.2 (42.5 to 61.7) | ||
| 40-49 | 1916 | 39.2 (34.2 to 44.4) | 768 | 55.9 (49.1 to 62.6) | 441 | 37.9 (31.2 to 45.0) | ||
| 50-59 | 2549 | 50.1 (46.3 to 53.8) | 1167 | 65.5 (62.4 to 68.5) | 771 | 39.2 (34.2 to 44.6) | ||
| 60-69 | 2317 | 51.8 (45.5 to 57.9) | 1171 | 66.3 (60.6 to 71.6) | 794 | 39.7 (36.1 to 43.4) | ||
| ≥70 | 1743 | 51.8 (46.8 to 56.7) | 885 | 64.9 (57.3 to 71.7) | 609 | 45.5 (40.3 to 50.8) | ||
| P for trend | — | <0.001 | — | 0.003 | — | 0.03 | ||
| Ethnicity: | ||||||||
| Han | 9076 | 44.0 (39.8 to 48.2) | 4210 | 49.1 (44.6 to 53.6) | 2647 | 49.5 (39.5 to 59.6) | ||
| Tibetan | 129 | 28.3 (14.9 to 47.2) | 48 | 43.4 (29.4 to 58.6) | 22 | 24.3 (24.3 to 24.3) | ||
| Uyghur | 233 | 34.2 (28.8 to 40.1) | 92 | 32.5 (10.3 to 66.9) | 45 | 36.9 (32.2 to 41.8) | ||
| Hui | 112 | 36.7 (23.7 to 51.9) | 36 | 66.7 (53.0 to 78.0) | 23 | 43.9 (40.9 to 47.0) | ||
| Zhuang | 222 | 27.1 (8.5 to 59.6) | 78 | 60.6 (40.6 to 77.6) | 55 | 40.8 (36.4 to 45.3) | ||
| P for difference | — | 0.047 | — | 0.86 | — | 0.12 | ||
| Region: | ||||||||
| South | 1003 | 45.4 (31.8 to 59.8) | 448 | 46.9 (39.3 to 54.7) | 219 | 67.0 (51.4 to 79.6) | ||
| North | 1820 | 39.0 (33.9 to 44.5) | 836 | 50.7 (37.6 to 63.7) | 548 | 51.3 (44.7 to 57.8) | ||
| East | 2216 | 41.8 (36.8 to 46.9) | 1012 | 39.8 (33.4 to 46.5) | 580 | 43.0 (32.3 to 54.4) | ||
| Central | 952 | 48.6 (42.2 to 55.1) | 529 | 49.0 (40.3 to 57.7) | 355 | 41.3 (40.7 to 41.9) | ||
| Southwest | 1374 | 33.2 (26.1 to 41.2) | 528 | 46.8 (37.7 to 56.2) | 355 | 53.3 (43.1 to 63.3) | ||
| Northwest | 1451 | 43.8 (35.6 to 52.3) | 612 | 58.0 (50.4 to 65.3) | 408 | 41.0 (31.7 to 51.0) | ||
| Northeast | 956 | 47.9 (40.3 to 55.6) | 499 | 61.4 (49.2 to 72.3) | 327 | 40.8 (30.9 to 51.5) | ||
| P for difference | — | 0.42 | — | 0.29 | — | 0.02 | ||
| Income per year (¥): | ||||||||
| ≤30 000 | 4652 | 43.1 (38.4 to 47.9) | 2089 | 50.7 (42.2 to 59.1) | 1301 | 51.7 (47.6 to 55.8) | ||
| >30 000 | 5012 | 43.1 (38.4 to 47.9) | 2325 | 49.6 (42.9 to 56.4) | 1473 | 45.5 (37.3 to 53.9) | ||
| P for difference | — | 0.59 | — | 0.69 | — | 0.12 | ||
| Education: | ||||||||
| Less than high school | 5708 | 44.3 (39.4 to 49.3) | 2562 | 46.6 (42.0 to 51.2) | 1669 | 44.7 (37.0 to 52.7) | ||
| High school and above | 4018 | 43.9 (39.3 to 48.6) | 1880 | 50.9 (45.8 to 56.0) | 1119 | 54.6 (44.1 to 64.8) | ||
| P for difference | — | 0.34 | — | 0.02 | — | 0.045 | ||
| Cigarette smoking: | ||||||||
| Current non-smoker | 6791 | 44.4 (39.6 to 49.3) | 3216 | 51.0 (45.4 to 56.7) | 2004 | 50.9 (39.8 to 62.0) | ||
| Occasional smoker | 304 | 38.5 (30.8 to 46.7) | 135 | 44.7 (37.7 to 52.1) | 84 | 29.5 (20.5 to 40.5) | ||
| Regular smoker | 2487 | 44.5 (34.9 to 54.5) | 1105 | 40.4 (35.6 to 45.3) | 704 | 42.9 (32.2 to 54.4) | ||
| P for trend | — | 0.98 | — | <0.001 | — | 0.01 | ||
| Family history of diabetes: | ||||||||
| Yes | 2623 | 59.3 (55.8 to 62.8) | 1640 | 56.4 (47.9 to 64.5) | 1080 | 49.2 (38.1 to 60.4) | ||
| No | 7138 | 36.7 (32.6 to 41.0) | 2815 | 43.6 (38.7 to 48.6) | 1709 | 46.8 (39.6 to 54.1) | ||
| P for difference | — | <0.001 | — | 0.23 | — | 0.9 | ||
| Body mass index: | ||||||||
| <25 | 4310 | 43.1 (38.0 to 48.3) | 2078 | 51.6 (42.6 to 60.5) | 1243 | 57.4 (51.8 to 62.9) | ||
| 25 to <30 | 4289 | 45.8 (39.5 to 52.3) | 1911 | 46.9 (40.2 to 53.8) | 1245 | 39.7 (30.6 to 49.5) | ||
| ≥30 | 1142 | 41.2 (36.8 to 45.7) | 462 | 51.0 (43.9 to 58.1) | 298 | 49.2 (39.7 to 58.8) | ||
| P for trend | — | 0.40 | — | 0.93 | — | 0.004 | ||
| Waist circumference (cm) | ||||||||
| Men ≥90, women ≥80 | 6466 | 44.1 (39.8 to 48.5) | 2924 | 48.8 (42.3 to 55.3) | 1869 | 42.3 (33.5 to 51.7) | ||
| Men <90, women <80 | 3250 | 42.3 (36.8 to 48.0) | 1505 | 50.0 (41.3 to 58.6) | 905 | 57.4 (49.4 to 65.0) | ||
| P for difference | — | 0.54 | — | 0.14 | — | 0.44 | ||
100=£12; €13; $14. Some values were missing in the total population: 989 for income, 295 for education, 50 for cigarette smoking, 29 for family history of diabetes, 167 for body mass index, and 317 for waist circumference.
In the multivariable logit models, male sex; older age; family history of diabetes; overweight and obesity; central obesity; each 10 mm Hg increase in systolic blood pressure; each 10 beats/min in heart rate; and raised total cholesterol, low density lipoprotein, and triglycerides levels were significantly associated with increased risks of total diabetes and prediabetes (table 5). Hui ethnic participants had a lower risk of total diabetes than ethnic Han participants.
Table 5.
Risk factors for total diabetes and prediabetes diagnosed by the American Diabetes Association criteria among adults living in mainland China. Data are odds ratio (95% confidence interval)
| Variable | Total diabetes | Prediabetes |
|---|---|---|
| Men | 1.29 (1.14 to 1.45) | 1.18 (1.08 to 1.28) |
| Age, per 10 year increment | 2.20 (2.05 to 2.36) | 1.54 (1.46 to 1.61) |
| Urban residence | 1.19 (0.87 to 1.62) | 1.03 (0.75 to 1.42) |
| Ethnicity (reference: Han): | ||
| Tibetan | 0.58 (0.14 to 2.37) | 0.93 (0.34 to 2.52) |
| Uyghur | 0.58 (0.26 to 1.30) | 0.31 (0.09 to 1.06) |
| Hui | 0.64 (0.45 to 0.90) | 1.13 (0.76 to 1.68) |
| Zhuang | 1.37 (0.78 to 2.39) | 1.19 (1.02 to 1.39) |
| Region (reference: South): | ||
| North | 1.24 (0.77 to 2.01) | 1.31 (1.03 to 1.66) |
| East | 1.06 (0.64 to 1.76) | 1.06 (0.82 to 1.37) |
| Central | 1.06 (0.67 to 1.69) | 1.25 (1.04 to 1.50) |
| Southwest | 1.48 (0.79 to 2.78) | 1.41 (0.98 to 2.02) |
| Northwest | 1.14 (0.72 to 1.80) | 1.14 (0.91 to 1.43) |
| Northeast | 0.74 (0.47 to 1.18) | 0.83 (0.57 to 1.19) |
| Family history of diabetes | 3.06 (2.74 to 3.42) | 1.27 (1.16 to 1.39) |
| Less than high school education | 1.11 (0.99 to 1.23) | 1.08 (0.96 to 1.21) |
| Income ≤¥30 000 RMB a year | 1.13 (1.01 to 1.27) | 1.03 (0.97 to 1.10) |
| Current smoking | 0.94 (0.85 to 1.05) | 0.97 (0.90 to 1.05) |
| Weight: | ||
| Overweight | 1.46 (1.26 to 1.69) | 1.25 (1.13 to 1.38) |
| Obesity | 2.62 (2.30 to 2.98) | 1.79 (1.56 to 2.06) |
| Central obesity | 1.49 (1.32 to 1.69) | 1.22 (1.11 to 1.35) |
| Systolic blood pressure per 10 mm Hg increase | 1.12 (1.10 to 1.15) | 1.05 (1.02 to 1.07) |
| Cholesterol | ||
| Total per 1 mmol/L | 1.19 (1.07 to 1.34) | 1.15 (1.05 to 1.25) |
| Low density lipoprotein per mmol/L | 1.12 (1.00 to 1.25) | 1.16 (1.06 to 1.27) |
| High density lipoprotein per mmol/L | 0.64 (0.53 to 0.78) | 0.89 (0.78 to 1.01) |
| Triglycerides per mmol/L | 1.16 (1.10 to 1.22) | 1.05 (1.00 to 1.11) |
| Heart rate, per increase of 10 beats/min | 1.21 (1.15 to 1.28) | 1.13 (1.09 to 1.16) |
100=£12; €13; $14.
Discussion
This large national survey indicated that 11.2% of adults (according to WHO criteria) or 12.8% of adults (ADA criteria including the addition of HbA1c) aged 18 and older living in China had diabetes in 2017. Thus the total number of patients with diabetes in mainland China is estimated to be 129.8 million (70.4 million men and 59.4 million women).
The prevalence of diabetes continues to increase worldwide. The Non-Communicable Disease Risk Factor Collaboration reported that the age standardised prevalence of adult diabetes is 10% in men and 8.8% in women.3 Between 1980 and 2014, the global prevalence of diabetes increased from 4.3% to 9.0% in men and from 5.0% to 7.9% in women.3 For the increase in the number of adults with diabetes, 28.5% was attributed to the increased prevalence, 39.7% to population growth and ageing, and 31.8% to both.3
The trend in the prevalence of diabetes in China is the same as that worldwide. The weighted prevalence of total diabetes diagnosed by the WHO criteria has been increasing from 9.7% in 2007 and 2010, to 10.4% in 2013, and to 11.2% in this study in 2017 (supplementary table 4).11 12 13 The prevalence of prediabetes was 35.2%, which was similar to the 35.7% reported in 2013 with the same ADA diagnostic criteria.11 In this study, a venous blood was used to test for HbA1c, and this method was accepted by most laboratories. These findings suggest that the prevalence of total diabetes in China maintains a trend of continuing growth without any plateau or inflection point.
Awareness, treatment, and control of diabetes
Although a continuing increase in self-reported diabetes was found, a decrease in the prevalence of newly diagnosed diabetes was noted under the same WHO diagnostic criteria (supplementary table 4), suggesting better awareness of diabetes than found in previous studies.11 12 13 This improved awareness could be attributed to the concerted effort of the China National Plan for Non-Communicable Diseases Prevention and Treatment in 2013-15 and the Chinese Diabetes Society’s Diabetes Prevention and Management Program (Bluelight Action) from 2010.19 The percentage control of diabetes in the 2017 survey was improved in comparison with the 2010 survey but remained the same as in the 2013 survey, which indicates that the healthcare system needs further strengthening and improvement. Thus systematically trained community physicians with the requisite skills and expertise in diagnosis, management, and treatment of diabetes are needed to improve its control.
Age and age specific difference in the prevalence of diabetes
The prevalence of total diabetes and prediabetes was extremely high in people over 50, as was found in previous studies using the same ADA diagnostic criteria.12 13 Compared with previous data, the prevalence of total diabetes in the younger population was relatively low and decreasing, which is inconsistent with the concept that diabetes has a tendency to be greater among the young in Asia.20 Increases in the prevalence of total diabetes, diagnosed by the WHO criteria, have resulted from an increased prevalence in those aged 40 and older, especially the population older than 50 (supplementary table 5, supplementary fig 4).11 12 13 The age specific difference might partially explain the phenomenon. Many patients with prediabetes in the younger population have more social and family responsibilities but poorer health awareness. As a result, effective lifestyle modification or medical treatments are lacking in this population. Additionally, people who remain free from diabetes and prediabetes after the age of 50 might have some advantages due to genetic, socioeconomic, and lifestyle factors, and will continue to be healthier with ageing. One study showed that prenatal exposure to famine markedly increased the risk of hyperglycaemia in two consecutive generations of adults living in China.21 Given that the Chinese famine of 1959–61 was among the most severe on record, the affected population comprises those with a current age of 50 and older, who showed a higher prevalence of diabetes and prediabetes.22 Whether or not prenatal exposure to famine affects diabetes remains controversial, and this needs to be investigated in future studies.23
Being overweight and obese
Obesity and being overweight are two important risk factors for diabetes, and changes in these factors could contribute to the changes in the prevalence of total diabetes.24 The prevalence of obesity increased from 5.7% in the 2010 survey to 6.3% in the 2017 survey.12 Waist circumference also increased significantly from 80.2 cm to 80.7 cm in the 2007 and 2010 surveys to 83.2 cm in the 2017 survey (no published data on the 2013 survey for waist circumference are yet available).11 12 An increase in mean values of waist circumference, and prevalence in general overweight and obesity, and central obesity from 2007 to 2017 could contribute to an increase in the prevalence of diabetes, although we found no changes in body mass index between the 2013 and 2017 surveys (supplementary table 4). Nevertheless, obesity is still an important risk factor for diabetes.
Genetic background and geography
It is generally accepted that genetic factors significantly influence the risk of developing diabetes. Substantial differences in genetic background are present among certain ethnic groups in mainland China. This study found that the prevalence of total diabetes and prediabetes in four ethnic groups was significantly different from that in the Han ethnic group. Compared with the Han ethnic group, the Tibetan ethnic group had a significantly lower prevalence of diabetes, which was consistent with the findings of a previous study.13 The difference might be associated with dietary patterns, altitude, economic development, and genetic factors.25 26 A wide variation in the prevalence of diabetes and prediabetes between the Hui and Uyghur ethnic groups was found in this study. The difference might be explained by factors such as genetic variation, although the two minority groups in northwest China lived among Muslims and shared similar lifestyles.27 28
Among the 31 provinces of mainland China, a large variation in the prevalence of diabetes and prediabetes was found. In Inner Mongolia, the prevalence of total diabetes was three times higher than that in Guizhou province. For prediabetes, Yunnan province had the highest prevalence, being three times higher than the lowest prevalence in Anhui province. Although few differences in the prevalence of total diabetes existed between regions, a considerable variation in awareness, treatment, and control of the disease was found. This difference might be associated with genetic factors, environmental factors, dietary patterns, level of medical care, lifestyle, and economic development levels. Therefore, appropriate allocation of medical resources should be a key priority for policymakers.
Urban-rural disparity
Variations based on urbanisation were found in the prevalence of total diabetes and prediabetes, and in awareness, treatment, and control of diabetes. Although the prevalence of total diabetes continues to be higher in urban than in rural areas, without a significant difference, the gap showed a decreasing trend compared with previous data.11 12 13 Compared with the national study conducted in 2013, the current prevalence of diabetes increased by 1% in urban areas but by 2.5% in rural areas under the same ADA diagnostic criteria. China has a large rural population, and sanitation is lacking, thus an increased prevalence of diabetes in rural areas will lead to increased diabetic complications. Given the higher prevalence of prediabetes, and lower awareness, treatment, and control of diabetes, in rural populations, a large number of people are at risk of developing diabetes without the implementation of effective preventive measures.
Strengths of the study
This study had several strengths. Firstly, this nationally representative epidemiological survey provided reliable data on the prevalence of total diabetes and prediabetes in mainland China by following a strict quality assurance and control protocol. We estimated the prevalence not only by using statistical methods to weight the results but also by recruiting individuals according to the composition by age and sex of each community and the urban-rural ratio, referring to the latest national census data, which may have provided more accurate results. Secondly, a similar study design and statistical methods to those used previously make our findings more comparable with historical data. Although lacking a high quality national cohort to estimate the incidence of diabetes, our findings could add value to the assessment of diabetes epidemics in mainland China. Lastly, our study also provides provincial and regional data on prevalence using the latest diagnostic criteria for diabetes.
Limitations of the study
Our study also had some limitations. Firstly, we did not obtain information about the physical activity, dietary patterns, or alcohol consumption of participants, which reduced our ability to explore some risk factors. Secondly, using levels of 6.5% for HbA1c to diagnose diabetes could be controversial since that level was set for the US population. Although geographic disparities might exist in HbA1c levels, a consistent threshold was preferred across the study to enable comparison. Thirdly, owing to our study design, we could not distinguish between type 1 and type 2 diabetes. Fourthly, we did not carry out repeat testing in people with abnormal glucose values over time. We categorised one reading as being synonymous with diabetes, which may overestimate the prevalence, compared with clinical practice.29 Lastly, non-residents, such as internal migrant workers, who account for more than a sixth of the nation’s total population in China and who were more likely to have a lower prevalence of diabetes, were not included in the study owing to the study design.30 31 These limitations could lead to an overestimation of diabetes in the population.
Conclusions and policy implications
In conclusion, the estimated prevalence of total diabetes and prediabetes diagnosed by the ADA criteria was 12.8% and 35.2%, respectively, among the Chinese population aged 18 and older between 2015 and 2017. The prevalence of total diabetes defined by the WHO criteria increased from 9.7% in 2007 to 11.2% in 2017 among adults living in China. Our findings indicate that diabetes is an important health problem in China. Continuing surveillance and effective control are needed to reduce its burden.
What is already known on this topic
The previous national survey of diabetes in China, conducted in 2013, found a diabetes prevalence of 10.9% and a prediabetes prevalence of 35.7% using the American Diabetes Association (ADA) diagnostic criteria
In 2013, China implemented major healthcare reform and several government prevention, screening, and management programmes for non-communicable diseases, which provided useful data to show whether these changes have affected the prevalence of diabetes
Such surveys generally use a single reading of glucose or HbA1c, rather than repeat readings which are typical in clinical practice, where clinical diagnosis is based also on patient symptoms
What this study adds
This nationally representative epidemiological survey indicated that the overall prevalence of diabetes in mainland China in 2017 was 12.8% using the ADA diagnostic criteria and 11.2% using World Health Organization criteria
The prevalence of diabetes in 2017 in China is higher than that found by previous national surveys in 2007, 2010, and 2013 using the same WHO diagnostic criteria
The findings suggest that diabetes is an important health problem in China
Acknowledgments
We thank Gang Hu (Pennington Biomedical Research Centre) for his careful revision and guidance of this manuscript; the participants of this study. For continuous support, assistance, and cooperation, we thank Jiang He and Chung-Shiuan Chen (Tulane University); Wei Gong, Chenling Fan, Hong Wang, Hongmei Zhang, Shuangning Ding, Xiaochen Xie and Tingting Liu (First Hospital of China Medical University); Caiping Li and Jian Huangfu (Affiliated Hospital of Inner Mongolia University); Nan Jin (Chinese PLA General Hospital); Wuquan Deng, Fang Deng (Third Military Medical University); Haicheng Zhou (First Affiliated Hospital of Dalian Medical University); Qingling Lu (Cardiovascular and Cerebrovascular Disease Hospital of Ningxia Medical University); Yunfeng Shen (Second Affiliated Hospital of Nanchang University); Guodong Liu (First Affiliated Hospital of Harbin Medical University); Junxiu Hou and Zhiqiang Zhang (Affiliated Hospital of Inner Mongolia Medical University); Hong Zhang (Second Xiangya Hospital); Xiaodong Mao, Qifeng Wang and Kun Wang (Nanjing University of Chinese Medicine); Yanping Wang (Fujian Medical University Union Hospital); Xiaojun Ma (First Affiliated Hospital of Zhengzhou University); Liheng Meng (First Affiliated Hospital of Guangxi Medical University); Weihua Linle and Tuanyu Fang (Hainan General Hospital); Xingjun Liu and Yanru Zhao (First Affiliated Hospital of Xi'an Jiaotong University); Lulu Chen, Jiaoyue Zhang and Hanyu Wang (Huazhong University of Science and Technology); Jingfang Liu and Songbo Fu (First Hospital of Lanzhou University); Qingguo Lv (West China Hospital); Chenglin Sun (First Hospital of Jilin University); Qiuming Yao and Ronghua Song (Shanghai University of Medicine and Health Science Affiliated Zhoupu Hospital); Tingting Chen (First Hospital of An Hui Medical University); Ben Niu (First People's Hospital of Yunnan province); Mingtong Xu and Feng Li (Sun Yat-sen Memorial Hospital); Lizhen Lan (First Hospital of Shanxi Medical University); Jun Yue and Jia Song (People’s Hospital of Tibet Autonomous Region); Yanan Li and Wei Luo (Qinghai Provincial People’s Hospital); Xiaoming Lou and Zhe Mo (Zhejiang Provincial Centre for Disease Control and Prevention); Nianchun Peng and Lixin Shi (Affiliated Hospital of Guiyang Medical University); Mian Wang, Qiuxiao Zhu and Lingling Yuan (Second Hospital of Hebei Medical University); Haiqing Zhang (Shandong Provincial Hospital affiliated with Shandong University); Yong Fan (First Affiliated Hospital of Xinjiang Medical University); Hongyan Wei (Tianjin Medical University General Hospital).
Web extra.
Extra material supplied by authors
Web appendix: Supplementary appendix
Contributors: YZL, DT, XS, GQ, YQ, HQ, BS, and HS contributed equally to the paper. ZS and WT are joint corresponding authors. ZS, WT, YZL, GQ, YQ, HQ, BS, HS, DT, and XS conceived and designed the study. ZS and WT supervised the study. ZS, WT, and YZL performed the statistical analysis. YZL, DT, XS, GQ, YQ, HQ, BS, HS, JB, BC, JD, LH, XL, YBL, HC, EL, CL, LL, XT, NT, GW, JAZ, YW, YX, LY, JY, LHY, YY, ZY, QZ, LZ, JZ, MZ, JJZ, GN, and YM conducted the epidemiological survey. All authors contributed to acquisition, analysis, or interpretation of data. ZS, WT and YZL drafted the manuscript. All authors revised the report and approved the final version before submission. ZS and WT are the guarantors and attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This work is supported by the Clinical Research Fund of the Chinese Medical Association (grant No 15010010589). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: funding for the project through a grant (15010010589) from the Chinese Medical Association; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: This study was approved by the medical ethics committee of China Medical University (2014-103-2).
Patient consent: All participants provided written informed consent after a thorough explanation of the research procedures.
Data sharing: No additional data available.
The lead authors (ZS and WT) affirm that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Dissemination to participants and related patient and public communities: The results of this research were reported in newsletters for study participants, and public lectures about disease prevention have been provided based on the results.
References
- 1. DeFronzo RA, Ferrannini E, Zimmet P, et al. International textbook of diabetes mellitus, two volume set. 4th ed Wiley-Blackwell, 2015. 10.1002/9781118387658 . [DOI] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211-59. 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet 2016;387:1513-30. 10.1016/S0140-6736(16)00618-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American Diabetes Association Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care 2018;41(Suppl 1):S13-27. 10.2337/dc18-S002 [DOI] [PubMed] [Google Scholar]
- 5. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 1998;15:539-53. [DOI] [PubMed] [Google Scholar]
- 6.Ministry of Public Health. Nutrition and health status among Chinese: result from the Fourth China Nutrition and Health Survey. Ministry of Public Health ed. Beijing; 2004. [Google Scholar]
- 7. Wu Y. Overweight and obesity in China. BMJ 2006;333:362-3. 10.1136/bmj.333.7564.362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhong XL. Diabetes mellitus survey in China. Chin Med J (Engl) 1982;95:423-30. [PubMed] [Google Scholar]
- 9. Pan XR, Yang WY, Li GW, Liu J, National Diabetes Prevention and Control Cooperative Group Prevalence of diabetes and its risk factors in China, 1994. Diabetes Care 1997;20:1664-9. 10.2337/diacare.20.11.1664 [DOI] [PubMed] [Google Scholar]
- 10. Gu D, Reynolds K, Duan X, et al. InterASIA Collaborative Group Prevalence of diabetes and impaired fasting glucose in the Chinese adult population: International Collaborative Study of Cardiovascular Disease in Asia (InterASIA). Diabetologia 2003;46:1190-8. 10.1007/s00125-003-1167-8 [DOI] [PubMed] [Google Scholar]
- 11. Yang W, Lu J, Weng J, et al. China National Diabetes and Metabolic Disorders Study Group Prevalence of diabetes among men and women in China. N Engl J Med 2010;362:1090-101. 10.1056/NEJMoa0908292 [DOI] [PubMed] [Google Scholar]
- 12. Xu Y, Wang L, He J, et al. 2010 China Noncommunicable Disease Surveillance Group Prevalence and control of diabetes in Chinese adults. JAMA 2013;310:948-59. 10.1001/jama.2013.168118 [DOI] [PubMed] [Google Scholar]
- 13. Wang L, Gao P, Zhang M, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA 2017;317:2515-23. 10.1001/jama.2017.7596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ji LN. Develop Chinese diabetes guidelines from clinical evidence in China—marking the tenth anniversary of the first edition of Chinese diabetes guidelines for type 2 diabetes. Chinese J Diabetes 2014;22:1-4. [Google Scholar]
- 15.International Diabetes Federation. IDF Diabetes Atlas, 8th ed. 2017. https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf.
- 16. Jia W, Tong N. Diabetes prevention and continuing health-care reform in China. Lancet Diabetes Endocrinol 2015;3:840-2. 10.1016/S2213-8587(15)00382-4 [DOI] [PubMed] [Google Scholar]
- 17.National Bureau of Statistics of China. Tabulation on the 2010 population census of the People’s Republic of China. 2010. http://www.stats.gov.cn/tjsj/pcsj/rkpc/6rp/indexch.htm.
- 18.World Health Organization. Obesity: preventing and managing the global epidemic. 1997. https://www.who.int/nutrition/publications/obesity_executive_summary.pdf. [PubMed]
- 19. Chan JC, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol 2014;2:969-79. 10.1016/S2213-8587(14)70144-5 [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Ning G. Diabetes: young-onset type 2 diabetes mellitus-a challenge for Asia. Nat Rev Endocrinol 2014;10:703-4. 10.1038/nrendo.2014.162 [DOI] [PubMed] [Google Scholar]
- 21. Li Y, He Y, Qi L, et al. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 2010;59:2400-6. 10.2337/db10-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Smil V. China’s great famine: 40 years later. BMJ 1999;319:1619-21. 10.1136/bmj.319.7225.1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li C, Lumey LH. Exposure to the Chinese famine of 1959-61 in early life and long-term health conditions: a systematic review and meta-analysis. Int J Epidemiol 2017;46:1157-70.. 10.1093/ije/dyx013 [DOI] [PubMed] [Google Scholar]
- 24. Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA 2003;289:76-9. 10.1001/jama.289.1.76 [DOI] [PubMed] [Google Scholar]
- 25. Liu L, Liu L, Ding Y, et al. Ethnic and environmental differences in various markers of dietary intake and blood pressure among Chinese Han and three other minority peoples of China: results from the WHO Cardiovascular Diseases and Alimentary Comparison (CARDIAC) Study. Hypertens Res 2001;24:315-22. 10.1291/hypres.24.315 [DOI] [PubMed] [Google Scholar]
- 26. Santos JL, Pérez-Bravo F, Carrasco E, Calvillán M, Albala C. Low prevalence of type 2 diabetes despite a high average body mass index in the Aymara natives from Chile. Nutrition 2001;17:305-9. . 10.1016/S0899-9007(00)00551-7 [DOI] [PubMed] [Google Scholar]
- 27. He G, Wang Z, Wang M, et al. Forensic ancestry analysis in two Chinese minority populations using massively parallel sequencing of 165 ancestry-informative SNPs. Electrophoresis 2018;39:2732-42. 10.1002/elps.201800019 [DOI] [PubMed] [Google Scholar]
- 28. Li J, Lou H, Yang X, et al. Genetic architectures of ADME genes in five Eurasian admixed populations and implications for drug safety and efficacy. J Med Genet 2014;51:614-22. 10.1136/jmedgenet-2014-102530 [DOI] [PubMed] [Google Scholar]
- 29. Burden F. High-risk populations should be screened for “pre-diabetes” and type 2 diabetes. Pract Diabetes Int 2013;30:233-237a. 10.1002/pdi.1782 . [DOI] [Google Scholar]
- 30.National Bureau of Statistics of China. Statistical communiqué of the People’s Republic of China on the 2012 national economic and social development. 2013. http://www.stats.gov.cn/english/NewsEvents/201302/t20130222_26962.html.
- 31. Bi Y, Wang L, Xu Y, et al. 2012 China Noncommunicable Disease and Risk Factor Surveillance in Migrant Workers Study Group Diabetes-related metabolic risk factors in internal migrant workers in China: a national surveillance study. Lancet Diabetes Endocrinol 2016;4:125-35. 10.1016/S2213-8587(15)00366-6 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary appendix