Figure 2.
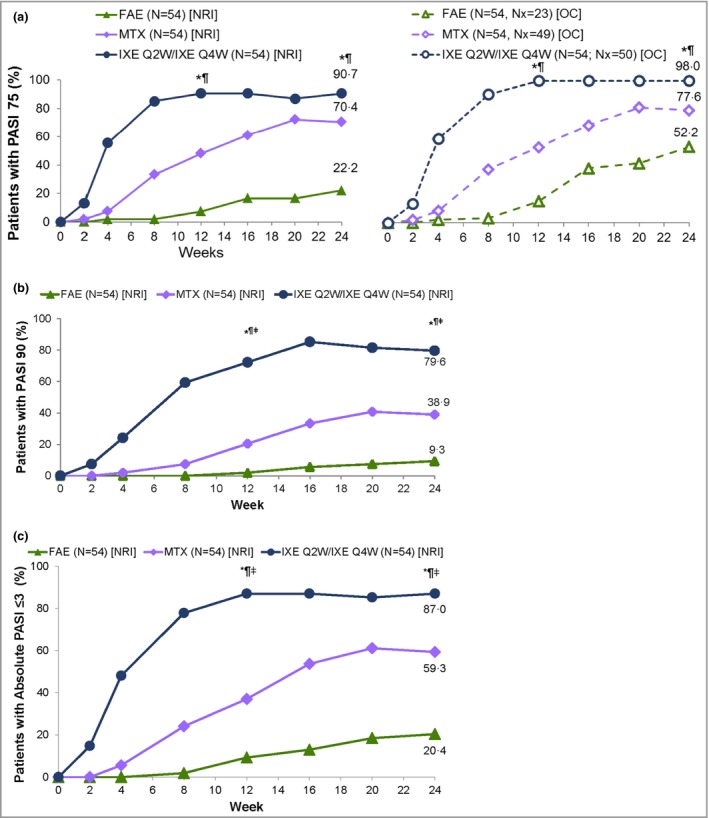
Patients with (a) ≥ 75% improvement in Psoriasis Area and Severity Index (PASI 75), (b) PASI 90 and (c) absolute PASI ≤ 3 responses at week 24 for the intention‐to‐treat (ITT) population with nonresponder imputation (NRI). FAE, fumaric acid ester; IXE, ixekizumab; MTX, methotrexate; Nx, number of patients with nonmissing data at week 24; OC, observed cases; Q2W, every 2 weeks; Q4W, every 4 weeks. *P < 0·05 IXE vs. FAEs, ¶ P < 0·05 IXE vs. MTX, ‡ddagger; P < 0·05 MTX vs. FAEs. Prespecified P‐values at 12 and 24 weeks are presented (Hochberg adjusted for PASI 75 and PASI 90).
