Figure 2.
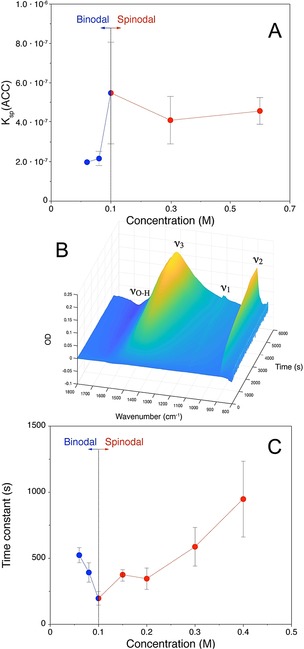
A) Evolution of ACC solubility K sp(ACC), determined by direct mixing of reactant solutions and potentiometric measurements, as a function of the initial reactant concentration of calcium and carbonate solutions. The significant step indicates entering the liquid–liquid spinodal regime. B) Temporal evolution of carbonate vibrational modes (ν1, ν2, ν3 with combination mode) and OH‐bending mode in water (νO‐H) after mixing of CaCl2 (0.3 m) and Na2CO3 (0.3 m) solutions at a 1:1 volume ratio. The water mode becomes negative with time as calcium carbonate forms and precipitates onto the internal reflection element of the ATR cell, thereby replacing water molecules. C) Dependence of the time constant (determined for the kinetics of calcium carbonate formation based on the time development of the ν2 carbonate out‐of‐plane vibrational band, Figure S5) on the initial calcium and carbonate concentration prior to mixing. The minimum value in the obtained time constant at an initial concentration of 0.1 m shows that the reaction kinetics become fastest at this point, as expected for the spinodal limit. Indeed, this point corresponds to the liquid–liquid spinodal limit determined in the potentiometric titrations within experimental certainty (cf. A).
