Figure 3.
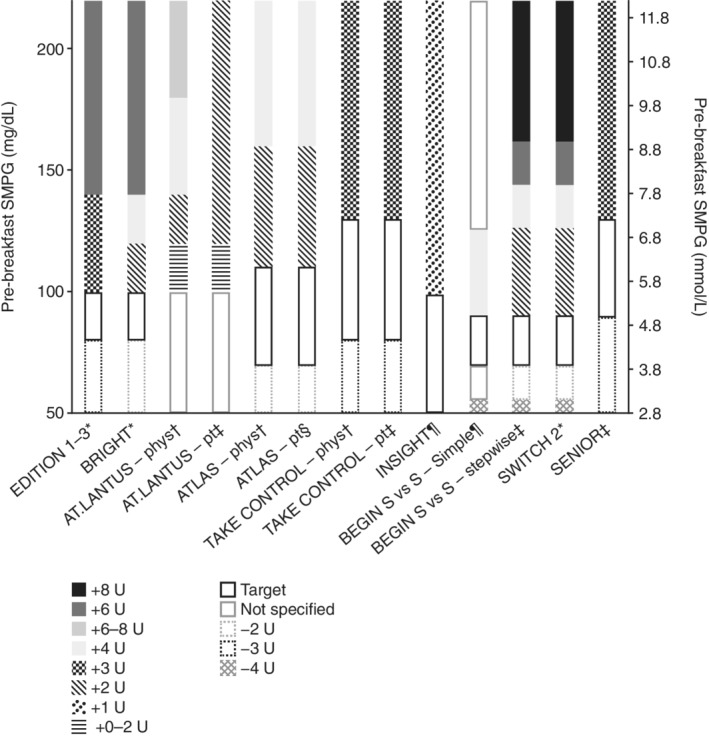
Titration algorithms in randomized controlled trials of basal insulins. For the EDITION 1–3 studies, if a blood glucose (BG) of <3.3 mmol/L (<60 mg/dL) was recorded, the dose could be reduced by 3 units (U) or at the investigator's discretion. In BRIGHT, if a BG of <4.4 mmol/L (<80 mg/dL) was recorded, the dose could be reduced by either 2 U or at the investigator's discretion. In ATLAS, if a BG of ≤3.1 mmol/L (≤56 mg/dL) was recorded, the dose was reduced at the physician's discretion. In TAKE CONTROL, the dose was reduced by 3 U in both the physician‐managed or self‐managed titration groups if either a BG of <4.4 mmol/L (<80 mg/dL) or ≥2 symptomatic or 1 severe hypoglycaemic event(s) in the preceding week were recorded. In SENIOR, the dose was reduced by 3 U if either a BG of <5.0 mmol/L (<90 mg/dL) or ≥2 symptomatic or 1 severe hypoglycaemic event(s) in the preceding week were recorded. Phys, physician‐managed titration group; pt, patient‐managed titration group; S vs. S, simple versus stepwise titration; SMPG, self‐measured plasma glucose.
*Doses were adjusted weekly; †doses were adjusted at every visit (AT.LANTUS: weekly during clinic visits or telephone contact visits; ATLAS: clinic visits at baseline, weeks 2, 6, 12, 16 and 24; telephone contact visits were made at weeks 1, 3, 4, 5, 8, 10 and 20; TAKE CONTROL: weekly for the first 8 weeks, biweekly until week 12, monthly until week 24); ‡doses were adjusted every 3 days; §doses were adjusted twice a week; ¶doses were adjusted daily
