Figure 2.
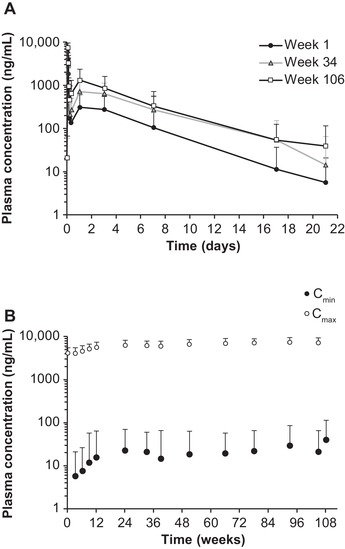
Plasma PK profiles for ALN‐18328 following intravenous administration of patisiran 0.3 mg/kg once every 3 weeks for 24 months of treatment in the OLE study. (A) Mean ALN‐18328 plasma concentration‐time profiles in weeks 1, 34, and 106. (B) Mean ALN‐18328 plasma Cmin and Cmax over time. Error bar represents standard deviation. ALN‐18328, patisiran drug substance (small interfering ribonucleic acid); Cmax, maximum concentration at EOI; Cmin, concentration at the end of the dosing interval; EOI, end of infusion; OLE, open‐label extension; PK, pharmacokinetic.
