Figure 5.
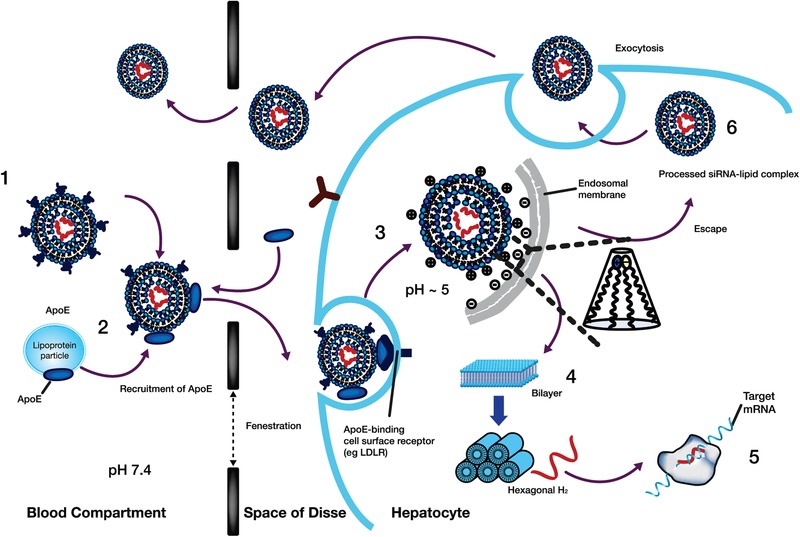
Proposed mechanism of liver uptake of patisiran LNP and release from the liver following intravenous administration. (1) After intravenous administration of patisiran, PEG2000‐C‐DMG dissociates from the LNP. (2) Removal of the PEG coating allows endogenous ApoE to associate with the LNP, facilitating uptake into hepatocytes via an ApoE‐dependent process. (3) On internalization via endocytosis, the ionizable DLin‐MC3‐DMA lipid is protonated (positively charged), as the pH decreases in the endosome. (4) The positively charged DLin‐MC3‐DMA lipid interacts with the negatively charged endosomal lipid, resulting in disintegration of the LNP, destabilization of the endosome membrane, and release of ALN‐18328 into the cytoplasm. (5) ALN‐18328 binds to RISC, leading to the targeted degradation of TTR mRNA and subsequent reductions in the target protein levels. (6) A proportion of internalized LNPs undergo exocytosis egress from late endosomes/lysosomes back into the circulation. ALN‐18328, patisiran drug substance (small interfering ribonucleic acid); ApoE, apolipoprotein E; IV, intravenous; LDLR, low‐density lipoprotein receptor; LNP, lipid nanoparticle; mRNA, messenger RNA; PEG2000‐C‐DMG, α‐(3′‐{[1,2 di(myristyloxy)proponoxy]carbonylamino}propyl)‐ω‐methoxy, polyoxyethylene; PEG, PEG2000‐C‐DMG; DLin‐MC3‐DMA, (6Z,9Z,28Z,31Z)‐heptatriaconta‐6,9,28,31‐tetraen‐19‐yl‐4‐(dimethylamino)butanoate; RISC, RNA‐induced silencing complex; siRNA, small interfering ribonucleic acid; TTR, transthyretin.
