Figure 1.
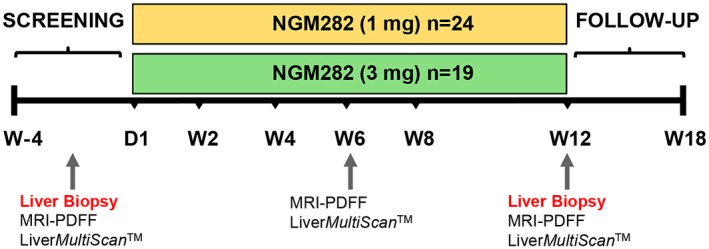
Study schema. Patients received NGM282 at a dose of 1 mg or 3 mg per day subcutaneously for 12 weeks. Liver biopsies were obtained at baseline and at week 12 (EOT). Liver fat content was measured by MRI‐PDFF at baseline, week 6, and week 12. Assessment of cT1 was performed at baseline, week 6, and week 12 by multimetric MRI LiverMultiScan. Serum markers of liver injury and fibrosis were measured by standard laboratory methods. Abbreviations: D, day; W, week.
