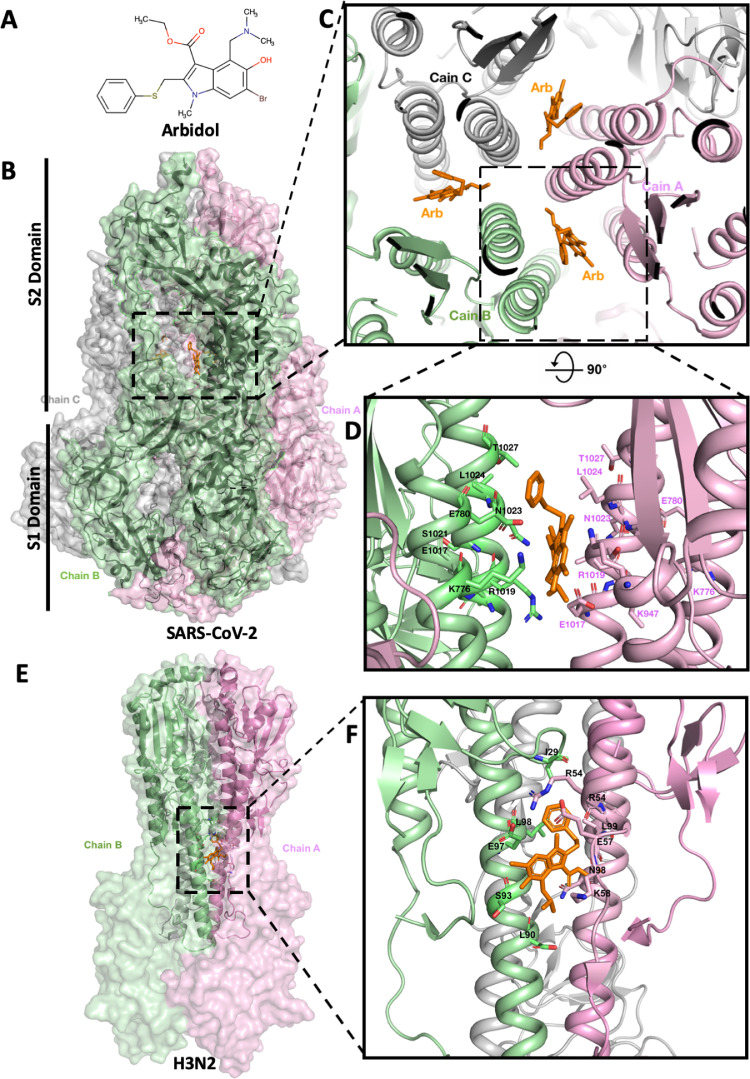
Fig. 1.
Arbidol binding site on SARS-CoV-2 spike glycoprotein. (A) Two-dimensional molecular structure of Arbidol. (B) Side view and overall view of the three-dimensional structure of Arbidol in complex with SARS-CoV-2 spike glycoprotein (surface model). The homotrimer structure of the spike glycoprotein is shown as a transparent surface (Chains A, B and C coloured in pink, green and grey, respectively), and the secondary structure in backbone traces. Arbidol is shown in orange. S1 and S2 domains are labelled. (C) Arbidol binding region in SARS-CoV-2 spike glycoprotein (top view). Three identical Arbidol binding sites are shown, viewed along the three-fold symmetry axis of the trimer. Individual monomers are coloured as above and labelled accordingly. (D) Cartoon model showing the Arbidol binding site and the key side chain residues (labelled accordingly) of SARS-CoV-2 spike glycoprotein involved in the interaction with Arbidol (orange). (E) Side view and overall view of the three-dimensional structure of Arbidol in complex with H3N2 haemagglutinin (HA) (surface model). Colour coding and labelling as above. (F) Cartoon model showing the Arbidol binding site and the key side chain residues (labelled accordingly) of H3N2 HA involved in the interaction with Arbidol (orange).