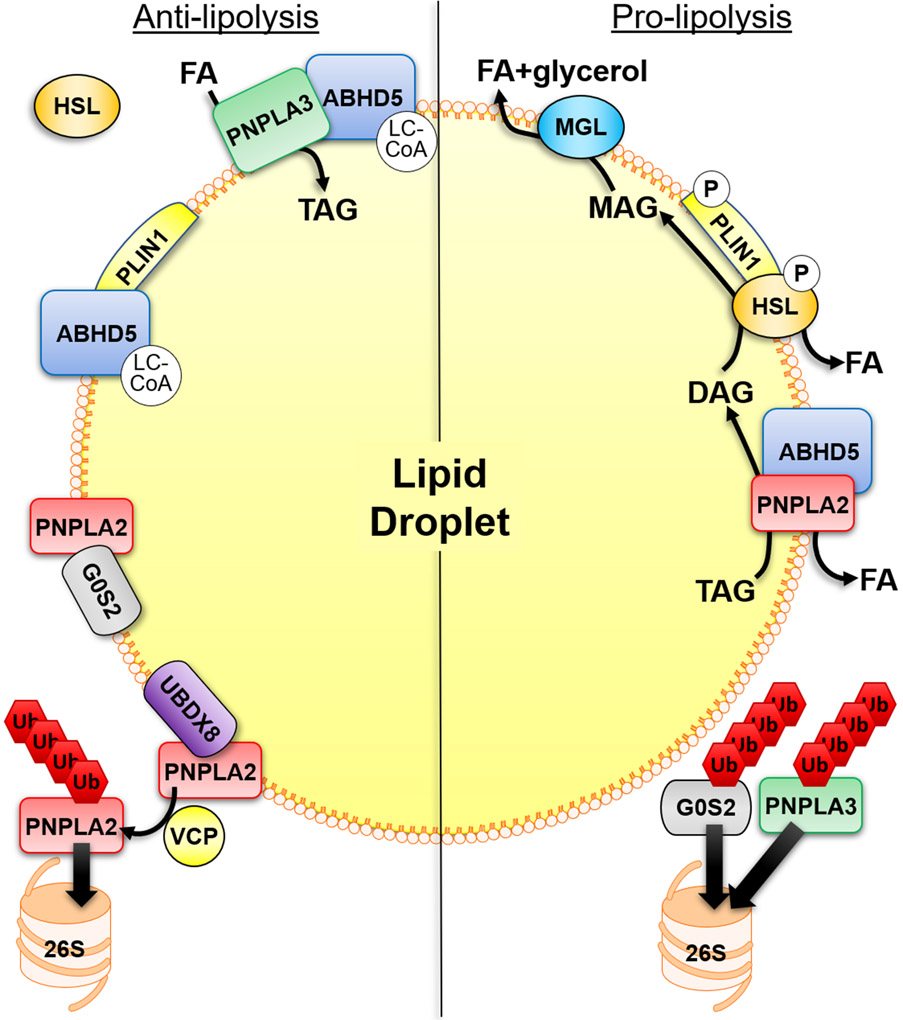
Figure 1. Mechanisms that regulate adipocyte lipolysis on the surface of lipid droplets.
Anti-lipolytic mechanisms of regulation include the binding of ABHD5 to PLIN1 which is regulated allosterically by ABHD5-sensing of long-chain acyl-CoA (LC-CoA). PNPLA2 hydrolytic activity can be inhibited by the interaction with G0S2 which is independent of its interaction with ABHD5. In addition, PNPLA3 can compete with PNPLA2 for binding to ABHD5, thereby sequestering ABHD5 away from PNPLA2. PNPLA3 can also function to sequester fatty acids (FAs) through lysophosphatidic acid acyltransferase or transacylase activity. LC-CoAs also allosterically regulate the interaction between PNPLA3 and ABHD5. UBDX8 can bind PNPLA2 to promote its segregation from the lipid droplet (LD) by VCP, followed by ubiquitination (Ub) and elimination by the proteasome (26S). In the basal state HSL is found mostly in the cytosol. Stimulatory signals lead to the phosphorylation (P) of PLIN1 to release ABHD5 which can bind PNPLA2, and phosphorylation of HSL promotes its trafficking to LDs and interaction with PLIN1. This leads to the sequential hydrolysis of TAG by PNPLA2, hydrolysis of DAG by HSL and hydrolysis of MAG by MGL to three FAs and glycerol. The elimination of G0S2 and PNPLA3 by the ubiquitin-proteasome system (UPS), through currently unknown mechanisms, would be pro-lipolytic.