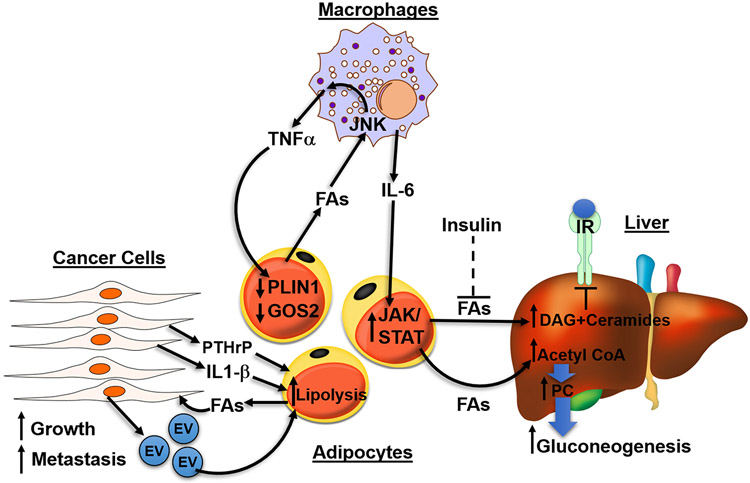
Figure 5. Adipocyte lipolysis is implicated in disease such as diabetes, fatty liver disease and cancer.
In diseased states, excessive fatty acids (FAs) activate signaling in surrounding cells to further increase lipolysis in adipocytes, forming a vicious, positive feedback loop. In obesity, adipocytes lose their ability to safely buffer FAs as TAGs, leading to the spillover of FAs which act upon macrophages through JNK signaling to release inflammatory cytokines TNFα and IL-6. Both cytokines function on adipocytes to further increase lipolysis. TNFα decreases PLIN1 and G0S2, inhibitors of PNPLA2, and IL-6 acts through JAK/STAT pathway to increase lipolysis. Under normal physiology, insulin functions to suppress lipolysis, however, in type 2 diabetes (T2D), the failure of insulin to suppress FA release from adipocytes (dashed line) results in excessive FA flux to the liver. FAs in the liver can form DAG and ceramides which act on PKC to decrease insulin sensitivity. Hepatic acetyl-CoA, generated from adipocyte FAs, allosterically upregulates pyruvate carboxylase (PC) to increase gluconeogenesis in the liver. Lastly, cancer cells secrete EVs and cytokine IL-1β increasing lipolysis of adipocytes in the tumor environment. Tumor-derived parathyroid-hormone-related protein (PTHrP) can promote the upregulation of lipolytic enzymes to promote cancer associated cachexia. Cancer cells use FAs generated from lipolysis as fuel to increase growth and invasiveness.