Figure 2: Schematic representation of MeHg-thiol exchange in the body.
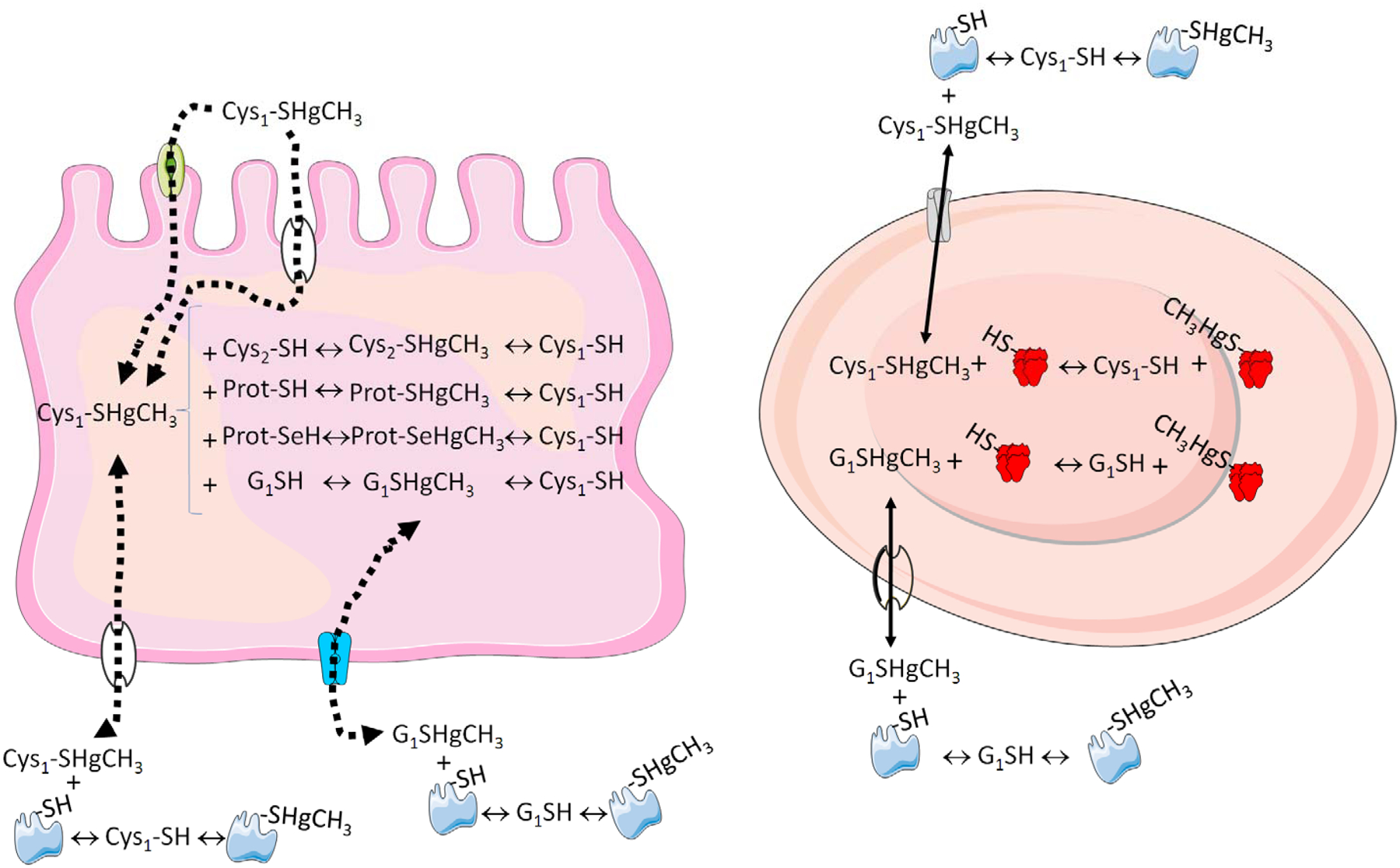
The absortion of MeHg-Cys complex (MeHg bound to a cysteine) in the intestine can be mediated by transporters or by exchange reactions. Inside the enterocyte (left part), the MeHg-Cys complex can exchange either with low molecular mass thiols (cysteine or glutathione, GSH) or with thiol-containing proteins. The complex can also exchange with selenol-containining proteins, i.e., selenoproteins (Prot-SeH). The low molecular mass sulfide-methylmercury complexes (GS-HgCH3 or Cys-S-HgCH3) can be transported to other cells or body fluids (e.g., interstitial fluid, capillary cells, which were omitted for the sake of clarity) and then can reach the plasma and blood cells (righ part). In the plasma, they can exchange with abundant proteins containing thiol (e.g. albumin) and can also be transported into the erythrocytes. Inside the erythrocytes, the low molecular mass thiols will delivery (exchange) the MeHg to hemoglobin and other thiol proteins, for instance, porphobilinogen synthase or aminolevulinate dehydratase (ALA-D, Rocha et al. 2012). The distribution of MeHg from plasma and erythrocytes to the tissues will involve the same kind of exchange reactions in the opposite direction (blood to tissue). In all the situations, the mobility of the low molecular mass complexes of MeHg-sulfides (MeHg-SG and MeHg-Cys) is expected to have a high facility to be redistributed than high molecular mass (thiol- or selenol-containing proteins).
