Figure 3: Exchange reaction between low molecular mass methylmercury-glutathione sulfide (MeHg-SG) with different thiol-containing proteins.
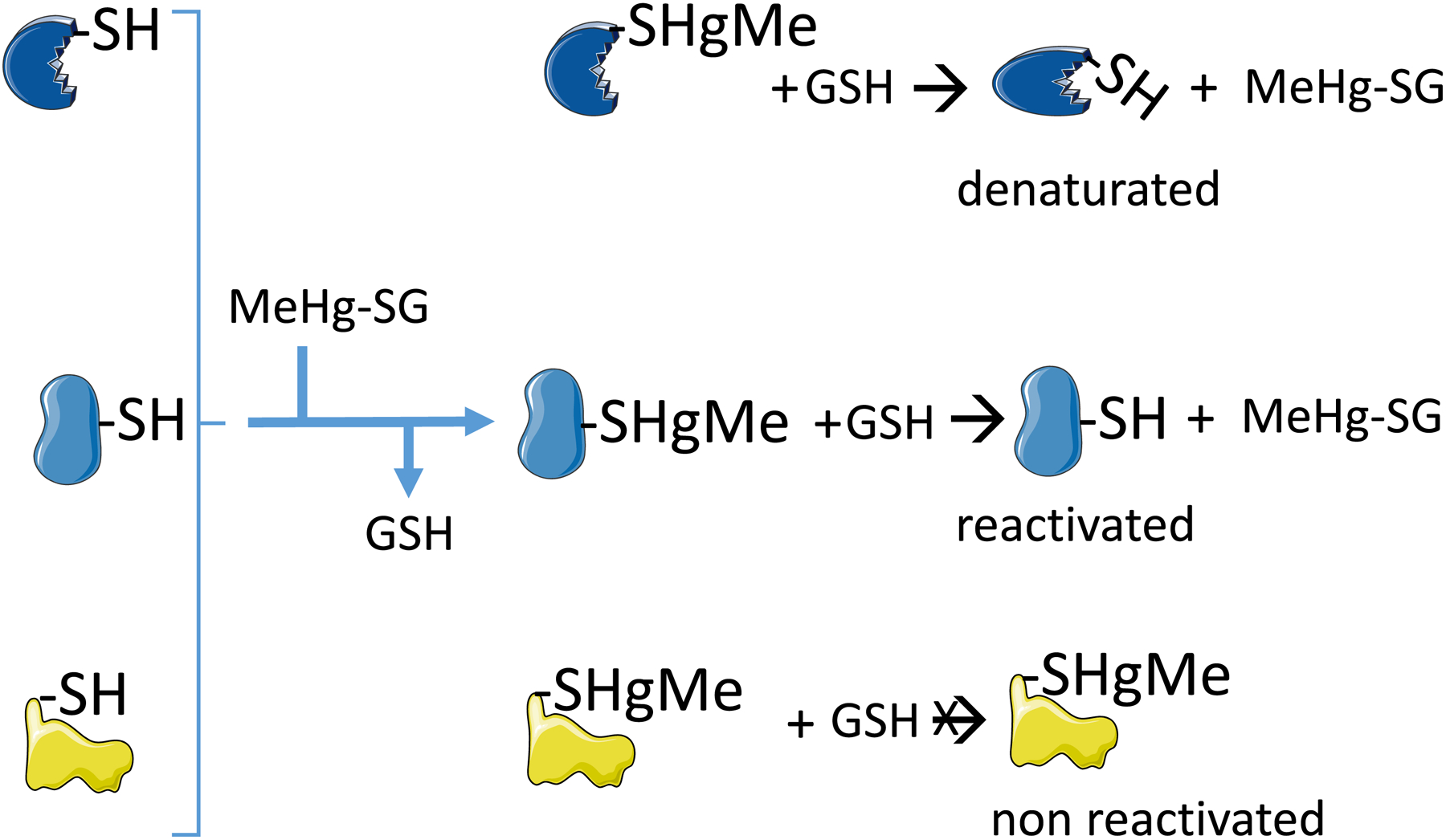
The first exchange (indicated by the reaction of the three proteins before the brace with GSH) results in the formation of three different protein-sulfide-methylmercury complexes. The protein-MeHg complexes are commonly inactive. The second set of exchange reactions can hypothetically reactivate the protein (represented by the second protein in the middle of the figure), or can release the denatured protein (i.e., the withdraw of the MeHg from the protein does not reestablish the activity, because the protein was denatured during the temporary interaction and is represented by the protein in the top of the figure). The reaction of MeHg with the protein thiol can cause a dramatic re-folding of the protein in such a way that MeHg-S-moiety will not be accessible to the medium and will not suffer the exchange reaction (represented by the third protein in the bottom of the figure).
