Scheme 1.
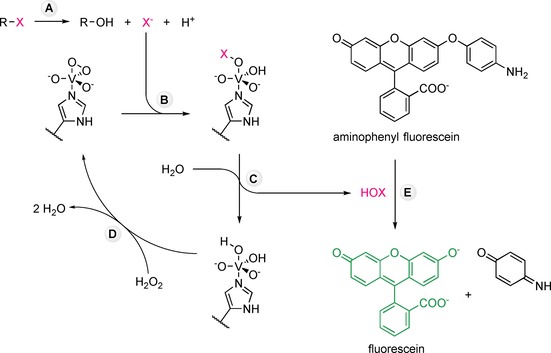
The principles behind the HOX assay for halides and dehalogenase activity. A) Haloalkane dehalogenases hydrolyse haloalkanes to the corresponding alcohols, protons and halide ions (X−). The halides formed are generally not very reactive but can be activated by a haloperoxidase‐catalysed two‐electron oxidation.18b, 19 In the case of vanadium‐dependent haloperoxidases the cofactor is a vanadate (V5+) ion coordinated by a conserved histidine residue. These enzymes are very stable because the cofactor cycles between the vanadate and peroxovanadate (oxidised) forms without changing the vanadium oxidation state. B) The halide ion and a proton react with the peroxovanadate cofactor, forming an intermediate that C) reacts with water to release the hypohalous acid (HOX). D) Hydrogen peroxide re‐oxidises the vanadate to peroxovanadate, completing the haloperoxidase catalytic cycle.18b E) Oxidation of aminophenyl fluorescein by the hypohalous acid results in the formation of fluorescein, a bright fluorescent dye that can be detected at nanomolar concentrations.
