Alemtuzumab is approved for the treatment of relapsing-remitting MS and is used off-label for patients with chronic lymphocytic leukemia and as induction and antirejection therapy in kidney transplant recipients.1 Guillain-Barré syndrome (GBS) or chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) complicating alemtuzumab treatment was reported in 9 patients with hematologic malignancy or MS.1–3 The risk of GBS or CIDP in solid organ transplant recipients treated with alemtuzumab is unknown.
Rabbit antithymocyte globulin (rATG) is another T cell–depleting drug used to treat acute kidney allograft rejection. Only 1 patient was reported who developed GBS after rATG treatment for aplastic anemia.4 We found no reports of GBS or CIDP complicating rATG treatment in kidney transplant recipients. Here, we investigated the frequency, type, and outcome of GBS and CIDP in a single-center cohort of kidney transplant recipients treated with either alemtuzumab or rATG.
Methods
Study design
A retrospective analysis was performed of a cohort of kidney transplant recipients who received either alemtuzumab (Campath, Sanofi-Genzyme, Cambridge, MA) or rATG (Thymoglobulin, Sanofi-Genzyme) between 2002 and 2018 in the Erasmus MC, Rotterdam. Alemtuzumab was administered as a single dose of 30 mg subcutaneously and rATG in a dose of 4 mg/kg and nog g/kg.
Statistical methods
Continuous variables are presented as median and interquartile ranges (IQRs). The 95% CIs were calculated with the modified Wald method. For statistical analysis, SPSS version 21 (SPSS Inc., Chicago, IL) was used.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Erasmus MC Medical Ethical Review Board (number 2018-1430).
Results
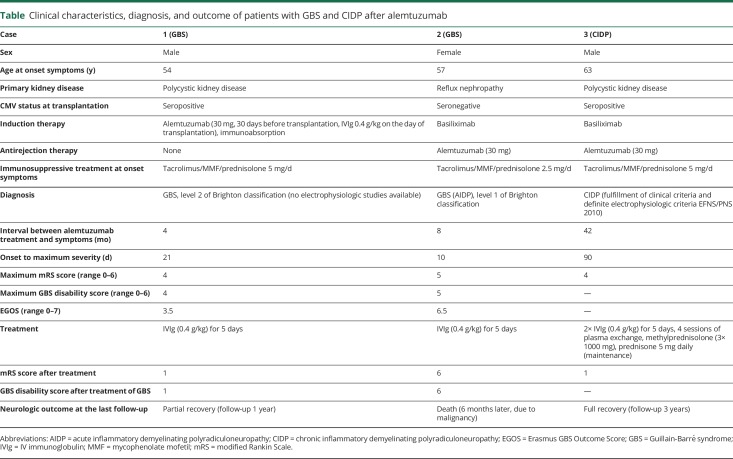
Between 2002 and 2018, 2,788 patients received a kidney transplant at our center. Alemtuzumab was administered to 143 (5.1%) patients and rATG to 108 (3.9%) patients. The total follow-up period of patients treated with alemtuzumab was 3.0 years (IQR 1.7–4.1 years) for a total of 444.3 person-years. A tacrolimus-based immunosuppressive regimen was given to 92% of patients. Three patients (2.1%, 95% CI 0.4%–6.3%) developed GBS or CIDP after alemtuzumab. Two patients fulfilled the diagnostic criteria for GBS, and 1 fulfilled the diagnostic criteria for CIDP. The clinical presentation and diagnostic findings of these patients are presented in the table. Laboratory tests, including clinical chemistry, serology, and virology, demonstrated no alternative diagnoses, and there was no recent Campylobacter jejuni or cytomegalovirus infection (PCR negative for cytomegalovirus). The total follow-up period for rATG-treated patients was 8.2 (IQR 6.3–11) years for a total of 829.4 person-years. Seventy-eight percent of patients received a tacrolimus-based immunosuppressive regimen. None of the patients treated with rATG (0%, 97.5% CI 0–4.1%) developed GBS or CIDP.
Table.
Clinical characteristics, diagnosis, and outcome of patients with GBS and CIDP after alemtuzumab
Discussion
This study shows that 2.1% of patients treated with alemtuzumab developed GBS or CIDP. This is higher than the incidence rate of these neuropathies in the general population and of kidney transplant recipients not treated with alemtuzumab.5–7 Secondary autoimmunity after alemtuzumab appears to be mainly B cell driven. A mismatched reconstitution of T and B cells after alemtuzumab can lead to an expansion of B cells in the absence of appropriate T-cell regulation. This may enable the escape of autoreactive B cells and production of pathogenic autoantibodies to self-antigens, which can lead to secondary autoimmunity, such as thyroiditis, idiopathic thrombocytopenic purpura, GBS, or CIDP.1
None of the patients treated with rATG developed GBS or CIDP. A possible explanation for the difference in the risk of developing these neuropathies with alemtuzumab is that the depletion of immune cells lasts longer after alemtuzumab.1 Alternatively, rATG may protect from GBS and CIDP.
Limitations of the current study are that we were unable to define the frequency of GBS and CIDP in kidney transplant recipients not treated with T cell–depleting therapy. Second, no causality between alemtuzumab and the risk of GBS or CIDP was demonstrated, and our findings may therefore relate to chance. Third, cytomegalovirus could have played a role in the development of GBS or CIDP because patients 1 and 3 were seropositive for cytomegalovirus at the time of transplantation. However, no signs of a reactivation were observed at the time the patients were diagnosed with GBS and CIDP. Fourth, we cannot exclude that the increased incidence of GBS and CIDP among alemtuzumab-treated patients may relate to the fact that in this group, more patients used tacrolimus as maintenance immunosuppression compared with the rATG cohort. Fifth, this observation is based on kidney transplant recipients who have several reasons to have an underlying neuropathy (i.e., renal insufficiency and diabetes mellitus), and it is uncertain whether it is also applicable to patients with MS.
In conclusion, alemtuzumab therapy in kidney transplant recipients may be associated with the development of GBS and CIDP. Clinicians should be alert for these neurologic complications in kidney transplant recipients treated with alemtuzumab.
Appendix. Authors
Study funding
No sources of funding were used in the preparation of this manuscript.
Disclosure
DA Hesselink has received grant support, lecture, and consulting fees from Astellas Pharma and Chiesi Pharmaceuticals, a lecture fee from Hikma Pharma, and grant support from Bristol-Myers Squibb. PA van Doorn has received grant support from Baxter, Sanquin Plasma Products, and Grifols. MvdH has received grant lecture and consulting fees from Astellas Pharma, Amgen, Genzyme, MSD, Roche, Sanofi, and Vifor Pharmaceuticals. BC Jacobs has received grant support from Annexon Biosciences, Baxter, CSL Behring, Hansa Biopharma, and Grifols. The other authors declare no conflicts of interest. Go to Neurology.org/NN for full disclosures.
References
- 1.van der Zwan M, Baan CC, van Gelder T, Hesselink DA. Review of the clinical pharmacokinetics and pharmacodynamics of alemtuzumab and its use in kidney transplantation. Clin Pharmacokinet 2018;57:191–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avivi I, Chakrabarti S, Kottaridis P, et al. . Neurological complications following alemtuzumab-based reduced-intensity allogeneic transplantation. Bone Marrow Transpl 2004;34:137–142. [DOI] [PubMed] [Google Scholar]
- 3.Hradilek P, Woznicova I, Slonkova J, Lochmanova A, Zeman D. Atypical acute motor axonal neuropathy following alemtuzumab treatment in multiple sclerosis patient. Acta Neurol Belg 2017;117:965–967. [DOI] [PubMed] [Google Scholar]
- 4.Kaya B, Davies CE, Oakervee HE, et al. . Guillain Barre syndrome precipitated by the use of antilymphocyte globulin in the treatment of severe aplastic anaemia. J Clin Pathol 2005;58:994–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willison HJ, Jacobs BC, van Doorn PA. Guillain-Barre syndrome. Lancet 2016;388:717–727. [DOI] [PubMed] [Google Scholar]
- 6.Ostman C, Chacko B. Guillain-Barre syndrome post renal transplant: a systematic review. Transpl Infect Dis 2019;21:e13021. [DOI] [PubMed] [Google Scholar]
- 7.Echaniz-Laguna A, de Seze J, Chanson JB. Chronic inflammatory demyelinating polyradiculoneuropathy in solid organ transplant recipients: a prospective study. J Neurol Neurosurg Psychiatry 2012;83:699–705. [DOI] [PubMed] [Google Scholar]