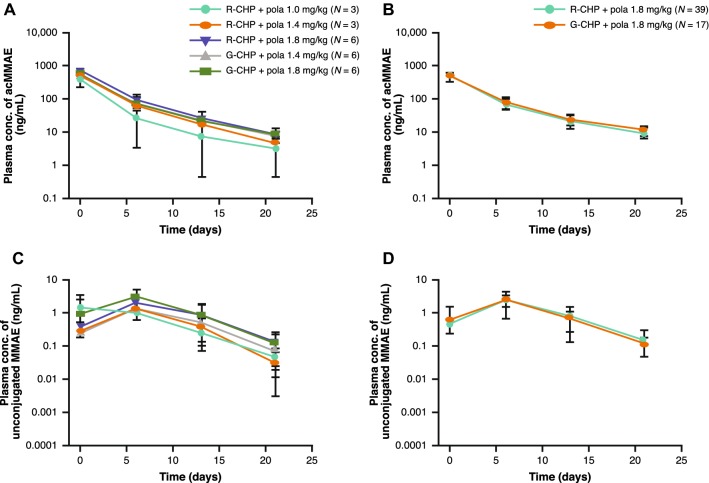
Fig. 1.
Mean (SD) cycle 1 plasma/serum concentration time profiles of pola by study phase and treatment in patients receiving pola 1.0–1.8 mg/kg in combination with R-CHP, or pola 1.4–1.8 mg/kg in combination with G-CHP. Dose-escalation arms are provided in a, c, with dose expansion arms in b, d. Pola analytes include acMMAE (a, b) and unconjugated MMAE (c, d). acMMAE antibody-conjugated MMAE, conc concentration, G-CHP obinutuzumab, cyclophosphamide, doxorubicin, and prednisone, MMAE monomethyl auristatin E, pola polatuzumab vedotin, R-CHP rituximab, cyclophosphamide, doxorubicin, and prednisone, SD standard deviation