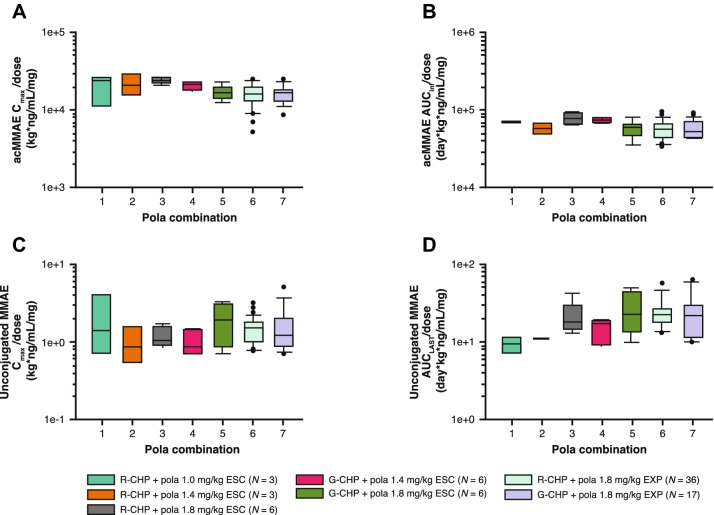
Fig. 2.
Comparison of dose-normalized exposure of pola by Cmax (a, c) or AUC (b, d) within cycle 1 across dose-escalation and expansion arms in patients with B-NHL or DLBCL receiving pola 1.0–1.8 mg/kg + R-CHP or pola 1.4–1.8 mg/kg + G-CHP. Pola analytes include acMMAE (a, b) and unconjugated MMAE (c, d). Vertical boxplots include the median, 10th, 25th, 75th, and 90th percentiles as vertical boxes with error bars for each respective cohort and outliers (black bulleted circles) plotted as single point. acMMAE antibody-conjugated MMAE, AUCinf area under the concentration–time curve from 0 to infinity, AUClast area under the concentration–time curve from 0 until the last measurable time point, B-NHL B-cell non-Hodgkin lymphoma, Cmax maximum concentration, DLBCL diffuse large B-cell lymphoma, ESC dose-escalation phase, EXP expansion phase, G-CHP obinutuzumab, cyclophosphamide, doxorubicin, and prednisone, MMAE monomethyl auristatin E, pola polatuzumab vedotin, R-CHP rituximab, cyclophosphamide, doxorubicin, and prednisone