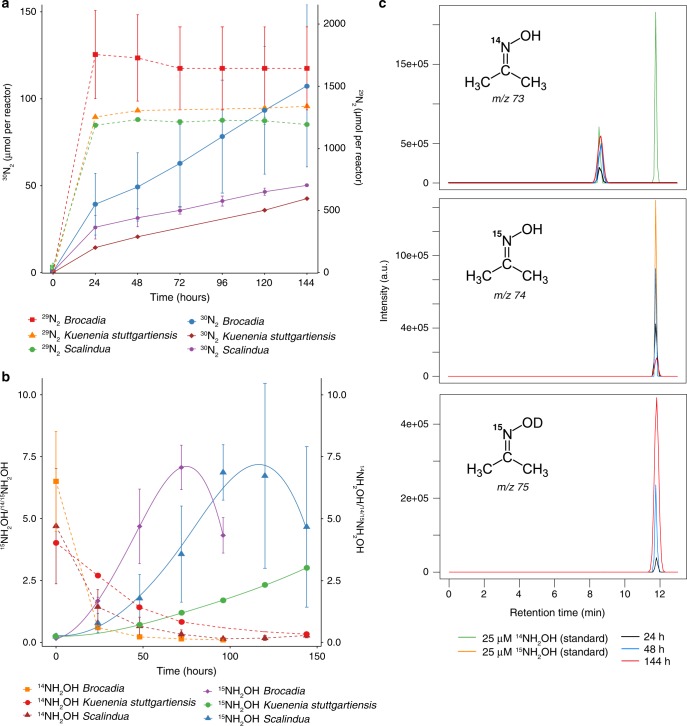
Fig. 3. Mechanism of NH4+ oxidation with extracellular electron transfer.
a Time course of the anaerobic oxidation of 15NH4+ to 29N2 and 30N2. The single-chamber microbial electrolysis cells (MECs) with mature biofilm on the working electrodes operated at 0.6 V vs. SHE were fed with 4 mM 15NH4+ and 1 mM 14NO2−. Under these conditions, anammox bacteria will consume first the preferred electron acceptor (i.e., 14NO2−) and form 29N2 and then the remaining 15NH4+ will be oxidized to the final product (30N2) through the electrode-dependent anammox process. NO and N2O were not detected throughout the experiment. Results from triplicate MEC reactors are presented as mean ± SD. b Determination of NH2OH as the intermediate of the electrode-dependent anammox process. The MECs with mature biofilm on the working electrodes operated at 0.6 V vs. SHE were fed with 4 mM 15NH4+ and 2 mM 14NH2OH. Under these conditions, anammox bacteria would preferentially consume the unlabeled pool of hydroxylamine (i.e., 14NH2OH), leading to the accumulation of 15NH2OH due to the oxidation of 15NH4+. Samples were derivatized using acetone, and isotopic ratios were determined by gas chromatography mass spectrometry (GC/MS). Results from triplicate MEC reactors are presented as mean ± SD. c Ion mass chromatograms of hydroxylamine derivatization with acetone. The MECs with mature biofilm (Ca. Brocadia) on the working electrodes operated at 0.6 V vs. standard hydrogen electrode (SHE) were fed with 4 mM 15NH4+ and 10% deuterium oxide (D2O). The mass to charge (m/z) of 73, 74, and 75 corresponds to derivatization products of 14NH2OH, 15NH2OH, and 15NH2OD, respectively, with acetone determined by GC/MS. Twenty microliters of 14NH2OH and 15NH2OH were used as standards. The 73 m/z (top) at a retention time of 8.6 min arises from the acetone used for derivatization. The 75 m/z (bottom) accumulation over the course of the experiment indicates that the oxygen used in the anaerobic oxidation of ammonium originates from OH− of the water molecule.