Table 1.
Formation of piperazinyl amide 4: selected optimization reactions.
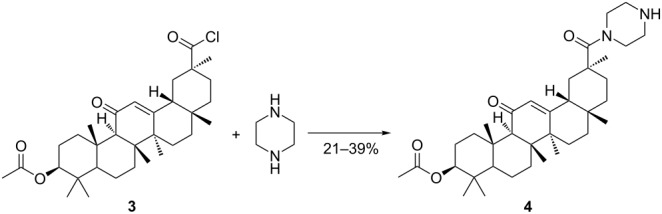 | ||
| entry | conditionsa | yield of 4b |
| 1 | piperazine (3 equiv), 0 °C | 21% |
| 2 | piperazine (6 equiv), 0 °C | 28% |
| 3 | piperazine (10 equiv), 0 °C | 33% |
| 4 | compound 3 (in 30 mL DCM) was added dropwise to the solution of piperazine (10 equiv), 0 °C | 36% |
| 5 | compound 3 (in 100 mL DCM) was added dropwise to the solution of piperazine (10 equiv), 0 °C | 39% |
| 6 | compound 3 (in 100 mL DCM) was added dropwise to the solution of piperazine (10 equiv), 25 °C | 32% |
aReaction performed on a 0.90 mmol scale of acyl chloride 3. bIsolated yield.
