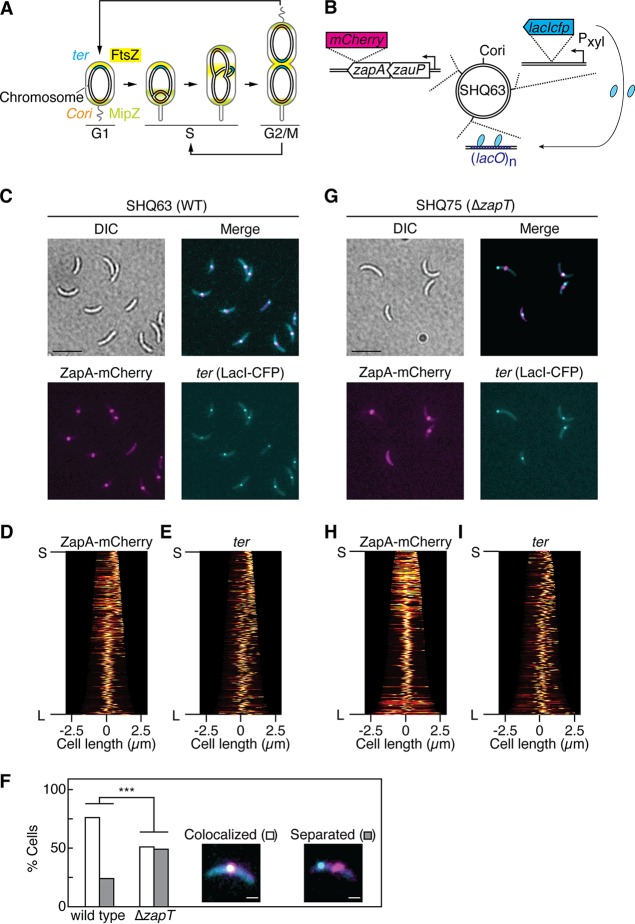
FIG 1.
The replication terminus region of the C. crescentus chromosome colocalizes with the divisome in a ZapT-dependent manner. (A) The cell cycle of C. crescentus. Localizations of the origin of replication (Cori, orange), the terminus (ter, cyan), FtsZ (yellow), and MipZ (green) at distinct cell cycle stages (G1, S, G2/M) are shown schematically. (B) Schematic representation of FROS-ter, which consisted of three modules: LacI-CFP expressed from the xylose-dependent promoter (Pxyl), (lacO)n repeats integrated near the terminus, and a zapA-mCherry fusion replacing the native zapA gene. The relative positions of the individual modules are shown schematically on the circular C. crescentus genome. (C to I) Analyses of FROS-ter in wild-type and ΔzapT mutant strains. SHQ63 (wild-type [WT]) cells (C to E) and SHQ75 (ΔzapT) cells (G to I) grown in M2G medium were analyzed using fluorescence microscopy. Representative differential interference contrast (DIC) images and fluorescent images of ZapA-mCherry and LacI-CFP are shown. Scale bar (C and G), 5 μm. Demographs of ZapA-mCherry (D and H) and LacI-CFP (E and I) expression were generated using Oufti software. For cells with a unipolar ZapA-mCherry focus, the ZapA-marked cell pole was defined as a new pole. “S” and “L” indicate the shortest and longest cells, respectively. (F) The percentages of cells in which the distance between the LacI-CFP focus and ZapA-mCherry focus was ≤5 pixels were plotted as “colocalized” (n > 200). ***, confidence level of >99.9% by a z-test.