Figure 10.
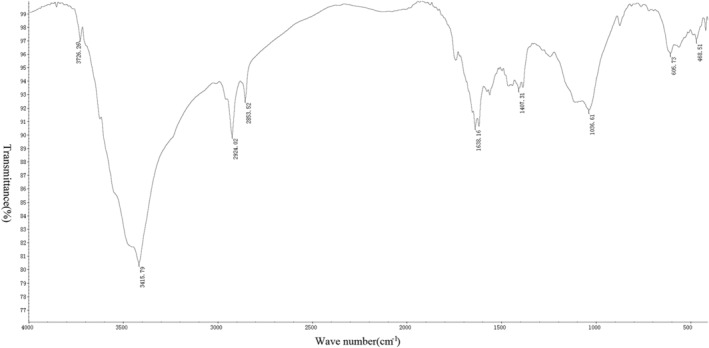
Infrared spectrum of acetone group: the small absorption peak at 605 cm−1 was caused by the asymmetric bending vibration of the O─P─O bond in PO4 3−. The strong absorption peak at 1036 cm−1 was the asymmetric stretching vibration peak of the P─O bond in PO4 3−. Asymmetric stretching vibration of CO3 2− could be observed at 1407 cm−1, indicating that the bone contains carbonate. The bending vibration peak of hydroxyl groups in water appeared at 1638 cm−1, indicating that the bone still contained a certain amount of moisture, while the strong absorption peak generated by hydroxyl groups in hydroxyapatite appeared at 3415 cm−1. There were two absorption peaks at 2853 and 2924 cm−1, which were mainly formed by the C‐H stretching vibration in saturated fatty acids, which proved that the bone in the acetone group still contains a small amount of fat.
