Figure 1. Regulation of eIF2B and the Integrated Stress Response (ISR).
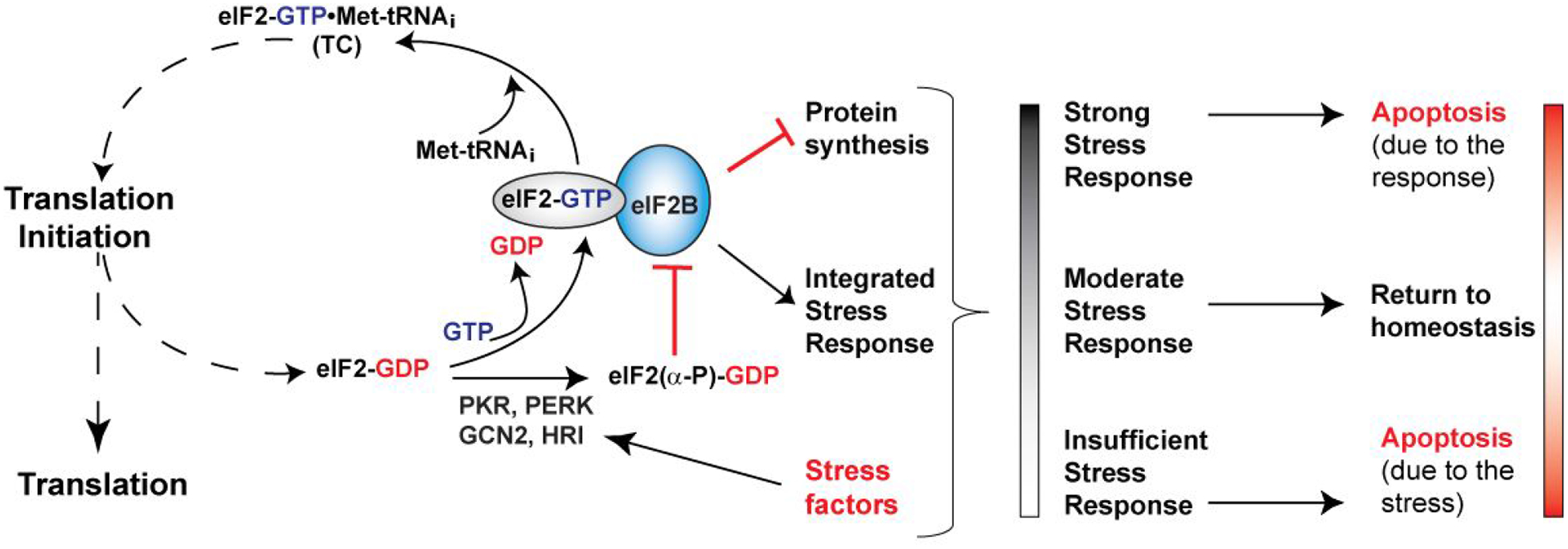
eIF2 brings the Met-tRNAi to the ribosomal translation initiation complex, in the form of the eIF2-GTP•Met-tRNAi ternary complex (TC). Upon start codon recognition, eIF2 hydrolyzes GTP, and eIF2-GDP is released. eIF2B catalyzes nucleotide exchange and Met-tRNAi binding to form a new TC. Phosphorylation of the α-subunit of eIF2 by several stress-activated kinases turns eIF2-GDP from substrate into an inhibitor of eIF2B. Inhibition of eIF2B activity triggers the ISR, which involves both pro-apoptotic and pro-survival pathways. The ultimate fate of the cell depends on the interplay between the stress, the pro-survival and pro-apoptotic branches of the ISR, and other stress responses in the cell. The stress response is usually proportional to the stress and self-contained through negative feedback. In the absence of adequate stress response, the stress factors can cause cell damage and death. At the opposite end of the spectrum, stress response that is too strong and/or prolonged can itself cause apoptosis.
