Figure 2.
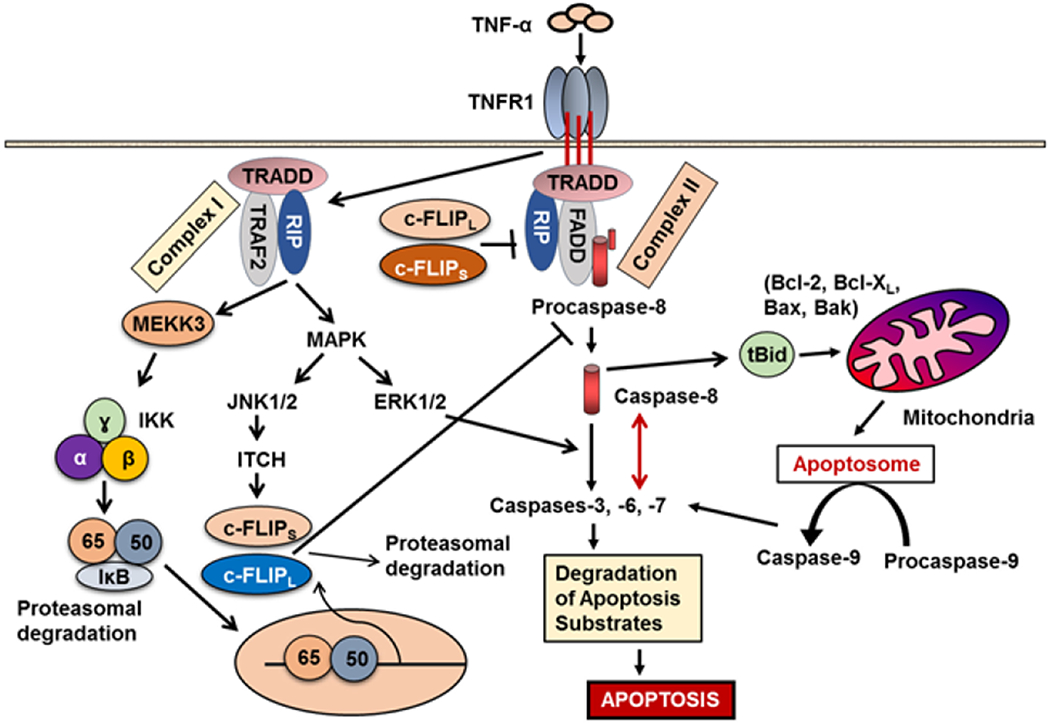
Death receptor and mitochondrial apoptosis signaling pathways and blocking TNFR1-mediated apoptosis by c-FLIP. TNF-α binds to its receptor TNFR1, which results in formation of Complex I containing TNFR1, TRADD, TRAF2 and RIP. Complex I mediates the NF-κB activation pathway and occurs through the MEKK3-IKK-IκB-NF-κB cascade, leading to the expression of c-FLIPL and c-FLIPS isoforms. TNF-α treatment through Complex I also activates JNK and ERK through the MAPK signaling pathway. The ubiquitin-E3-ligase ITCH promotes the ubiquitylation and proteasomal degradation of c-FLIP isoforms. As a result of degradation, levels of c-FLIP protein isoforms decrease. Complex II consists of RIP, TRADD, FADD, and procaspase-8. Caspase-8 is autoactivated and activates caspases-3 and -7, there by triggering apoptosis. Caspase-8 induces cleavage of the proapoptotic protein Bid to truncated Bid (tBid) which activates the mitochondrial apoptosis pathway that involves the release of cytochrome c and Smac/DIABLO from the mitochondria. Cytochrome c binds to Apaf1 to activate caspase-9-mediated executor caspases (Modified and updated from Safa).
