Table 2.
Cross-functionalization experiments to examine the competitive reactivity of α-haloglycine esters 4a–c.[a–d]
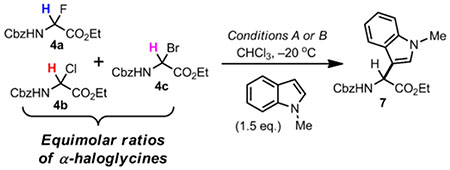 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Time (h) | 0 | 0.25 | 0.5 | 1 | 1.5 | 2.5 | 3.5 | 6 | 8 | 10 | |
| 1 Condition A Uncat. | α-haloglycine % consumption | 4a | 0 | 12 | 15 | 18 | 12 | 30 | 32 | 33 | 30 | 36 |
| 4b | 0 | 37 | 40 | 57 | 53 | 47 | 40[d] | 47 | 50 | 43 | ||
| 4c | 0 | 8 | 14 | 14 | 24 | 32 | 43 | 54 | 59 | 76 | ||
| % Yield (7) | 0 | 9 | 11 | 12 | 14 | 19 | 20 | 28 | 29 | 37 | ||
| 2 Condition B TA (10 mol%) | α-haloglycine % consumption | 4a | 0 | 18 | 15 | 18 | 15 | 15 | 21 | 21 | 15 | 27 |
| 4b | 0 | 33 | 50 | 43 | 50 | 47 | 53 | 67 | 40 | 37[d] | ||
| 4c | 0 | 27 | 49 | 49 | 57 | 59 | 70 | 70 | 73 | 86 | ||
| % Yield (7) | 0 | 20 | 23 | 25 | 29 | 32 | 36 | 40 | 43 | 45 | ||
Reactions performed on 0.5 mmol scale, conditions A: 4a–4c (1.0 eq.), conditions B: 4a–4c (1.0 eq.) with thiourea TA (10 mol-%).
The conversions were determined using 1H NMR with mesitylene as internal standard, from reaction aliquots in CHCl3 transferred in NMR tubes and adjusted to a mixture of CHCl3/CD3CN (2:1) at low temperature.
Average measurements are reported from triplicate experiments.
A bromide-chloride exchange is likely occurring through an external ion-return between the two glycinyl iminium ion-pairs 6b:6c (X = Br, Cl).
