Abstract
Purpose:
In a single-institution phase II study, we evaluated the safety of a five-day dose-equivalent neoadjuvant radiation therapy (RT) regimen for high-risk primary soft tissue sarcoma (STS).
Methods:
Patients received neoadjuvant RT alone (30 Gy in five fractions) to the primary tumor with standard margins. The primary endpoint was grade ≥2 late radiation toxicity. Major wound complications, local recurrences and distant metastases were also examined. In exploratory analysis, we evaluated germline biomarkers for wound toxicity and the effects of the study on treatment utilization.
Results:
Over two years, 52 patients were enrolled with median follow-up of 29 months. Seven of 44 evaluable patients (16%) developed grade ≥2 late toxicity. Major wound complications occurred in 16 of 50 patients (32%); a signature defined by 19 germline single nucleotide polymorphisms in miRNA binding sites of immune and DNA damage response genes, in addition to lower extremity tumor location, demonstrated strong predictive performance for major wound complications. Compared to the preceding two-year period, the number of patients treated with neoadjuvant RT alone at our institution increased three-fold, with a concomitant increase in the catchment area.
Conclusions:
A shorter five-day neoadjuvant RT regimen results in favorable rates of wound complications and grade ≥2 toxicity after two years follow-up. Five-day RT significantly increased utilization of neoadjuvant RT at our high-volume sarcoma center. With further validation, a putative germline biomarker for wound complications may guide safer RT utilization.
Keywords: soft tissue sarcoma, neoadjuvant therapy, radiation therapy, hypofractionation
INTRODUCTION
Radiation therapy (RT) significantly improves local control for patients with high-risk primary extremity and trunk soft tissue sarcomas (STS)7. Neoadjuvant RT is generally preferred due to its favorable toxicity profile, shorter course, and potential operative benefits1. Although widely considered a standard approach for high-risk STS, many patients do not receive RT, in part due to the difficulty of daily treatments for five or six weeks8,9.
More condensed RT regimens have been adopted in the treatment of several malignancies as radiation oncologists can more easily spare normal tissues with modern radiation techniques and image guidance3,10. There is also a biological rationale for this approach in sarcoma, a tumor that is less sensitive to smaller RT fraction sizes (lower α/β ratio)11. Although late toxicities are still a concern, these condensed RT regimens can be well tolerated with appropriate dosimetry12,13.
For STS patients who also receive neoadjuvant chemotherapy, a condensed (hypofractionated) form of neaodjuvant RT has been used at our institution for decades. This regimen of neoadjuvant chemoradiation (the “Eilber” protocol, 28 Gy over eight fractions with ifosfamide-based chemotherapy) demonstrated an actuarial local recurrence rate of 11% and 17% at three and six-years14,15. Another Polish study utilizing five-day neaodjuvant radiation (25 Gy over 5 fractions) for STS resulted in 19.1% rate of local recurrence at median follow-up of 35 months16. The risk of local recurrence in these two studies was higher than modern studies using standard fractionation (50 Gy over 25 fractions in five weeks), which have reported <10% local recurrence rates2,3,17,18. We hypothesized that this may be due to the lower biologically equivalent or effective dose used in both hypofractionation studies.
We initiated a prospective phase 2 study to evaluate the safety and toxicity of a five-day neoadjuvant RT regimen for STS that delivers 30 Gy over 5 fractions, a dose that may more closely mimic the biological effect of conventional 5-week RT(EQD2 = 50 Gy). This calculation is based on a presumed α/β = 4 for STS11,19, though this is a generalized estimate of a value that is more likely histology- and tumor-specific. Here, we report the feasibility, safety, and early onocologic outcomes of this prospective phase 2 study.
PATIENTS AND METHODS
Patients
The protocol for this prospective study (NCT012701153) was approved by the UCLA Institutional Review Board. Informed written consent was obtained from eligible patients with histologically confirmed STS of the extremity or trunk with planned neaodjuvant RT and surgery. The study was performed according to institutional regulations as well as ethical principles summarized in the Belmont Report. All patients were 18 or older and had Eastern Cooperative Oncology Group (ECOG) performance status 0 to 2. Exclusion criteria included evidence of distant metastases, planned neoadjuvant or adjuvant chemotherapy; prior RT to the area to be irradiated; and active treatment of a second malignancy.
Study Design and Treatments
Eligible patients were assigned to receive neaodjuvant RT followed by surgery 2 to 6 weeks later. Radiation CT and/or MRI simulation was performed with custom immobilization. The gross, clinical and planning target volumes were defined according to RTOG 06303: the gross tumor volume (GTV) was defined by T1 weighted MRI and CT. The clinical target volume (CTV) was defined as a margin of 3 cm in the longitudinal directions on the GTV and a 1.5 cm radial expansion on the GTV, in addition to any suspicious edema as seen on T2 weighted MRI, and cropped out of any uninvolved bone and non-adjacent muscle compartments. This clinical target volume (CTV) was then expanded to a planning treatment volume (PTV) using a 5 mm expansion. The PTV was cropped at least 2mm from the skin for superficial lesions unless there was skin involvement.
A dose of 6 Gy × 5 fractions (30 Gy) was delivered on consecutive days to at least 95% of the PTV. Intensity modulated (IMRT), 3D conformal, or electron planning techniques were used. Radiation plans were deemed acceptable if they met dosimetric parameters outlined in Supplementary Table S1. All patients underwent daily image guidance except two patients receiving electron RT. All surgeries were performed at UCLA by one of four dedicated sarcoma surgeons.
Assessments
The primary endpoint of this study was the rate of grade ≥ 2 radiation morbidity (fibrosis, lymphedema or joint stiffness) at median two-year follow-up (minimum one year). Fibrosis and joint stiffness were graded based on Radiation Therapy Oncology Group/EuropeanOrganisation for Research and Treatment of Cancer (RTOG/EORTC) criteria, and lymphedema was graded by Stern’s scale. Other secondary endpoints included acute toxicities as assessed by the frequency and severity of adverse events (AEs) using CTCAE v4.0 toxicity criteria, the rate of major wound complications, pathologic treatment effect, and the rate of local and distant recurrences. Major wound complications were defined as per established criteria from prospective clinical trials of extremity soft tissue sarcoma1,2. We also evaluated patient and physician-reported functional outcomes at baseline and 12, 18 and 24 months using the Toronto Extremity Salvage Score (TESS) and the Musculoskeletal Tumor Society (MSTS score), respectively20,21. Pathologic treatment effect was defined as the percentage of surgical tissue with hyalinization or necrosis relative to pre-treatment biopsy22. Time-to-event end points were measured from enrollment.
Patients were seen after the completion of radiation and prior to surgery by the radiation oncologist and/or the sarcoma surgeon. Patients were followed closely in the postoperative setting. The patients’ status was reviewed 3 months after surgery, and then at least every 6 months thereafter. The patients were followed both clinically and radiographically after treatment with CT or MRI of the primary site and CT of the chest at least every 6 months for the first two years and then at least annually in the third year. Patients with myxoid liposarcoma were also evaluated with CT of the abdomen and pelvis.
A subset of patients were consented to a parallel imaging study under IRB approval from May 2016 to June 2018. Diffusion-weighted images (DWI) were acquired up to four times for each patient using a 0.35T MR-guided radiotherapy machine (ViewRay, MRIdian, Mountain View, CA) including before the first fraction of treatment and and at least 14 days after RT but prior to surgical resection.
Statistical Analysis
The study was designed to evaluate the rate of grade ≥2 radiation morbidity (subcutaneous tissue fibrosis, joint stiffness, or edema at 2 years to mirror the initial design of RTOG-06303 with a target absolute improvement of 20% in the rate of grade ≥ 2 radiation-associated toxicity at two years compared to the historical neaodjuvant RT arm of the CAN-NCIC-SR2 study from 37% to 17%. Between May 2016 and May 2018, 52 patients with localized high-risk STS of the extremity or trunk were enrolled. Of these, 50 patients ultimately underwent neaodjuvant RT and surgery (Supplementary Figure S1, CONSORT diagram).
We examined whether major wound complications were related to relevant clinical variables, including tumor size, tumor site, tumor depth, time interval from radiation to surgery, and two radiation dosimetric variables using univariate logistic regression. Dosimetric variables included the maximum radiation dose (Gy) to the skin (minimum 0.5 cc volume), and volume of the skin (cc) receiving 12 Gy. Likelihood ratio test (LRT) was used to assess the significance of categorical variables.
Pathologic outcomes were reported with descriptive statistics. Differences in pre- and post-treatment tumor volumes by diffusion-weighted MRI were assed by paired t-test. Comparisons of average distance traveled and volume of patients between the two years prior to study enrollment and the study period were made using unpaired t-tests.
Exploratory Germline Biomarker Analysis
Genomic DNA from blood or saliva was analyzed for single nucleotide polymorphisms (SNPs) disrupting miRNA binding sites, promoter regions or coding sequences as previously identified23. Biomarkers in binding sites in genes involved in the immune system and DNA damage response, as well as promoters and coding sequences of miRNAs that regulated key genes known to be critical in the DNA damage or immune response were enriched in our analysis. We reduced to a final list of ~116 variants (see Supplemental Methods). We evaluated the relationship of this set of 116 SNPs with the incidence of major wound complications. We also included lower extremity tumor site as a variable as it was the only clinical variable associated with major wound complications. The association between this panel of potential germ-line biomarkers and tumor site with wound toxicity was assessed using four classifiers: classification trees (CT)24, random forests (RF)25, boosted trees (BT)26, and LASSO-regularized logistic regression (LASSO-LR)27, which were fit in R (version 3.6.0)28.
Retrospective analysis of neaodjuvant RT patients prior to clinical trial period
Patients treated with neaodjuvant RT alone prior to surgical resection during the two-year period (May 2014 to May 2016) before study initiation were extracted from the facility electronic health record system. Distance to facility was calculated using the patients’ residential zip codes and the facility zip code in R package ggmap29.
RESULTS
Patient characteristics and accrual
Patient clinical and pathologic details and demographics are summarized in Table 1 and Supplementary Figure S2. The study enrolled patients across a broad age spectrum, including five patients between age 80 and 90, three of whom had ECOG performance status of 2. With the exception of one patient’s tumor, all were intermediate- or high-grade. Tumor size among enrolled patients was heterogenous (1.2 – 28 cm), and twelve patients had tumors >10 cm. Twelve patients (24%) received neoadjuvant RT prior to re-resection for gross (n=9, 18%) or microscopic (n=3, 6%) residual disease. The median time between completion of RT and surgery was 28 days (range 14 – 55). Nine of 50 patients (18%) had initial R1 resection, of whom 5 underwent R0 re-resection.
Table 1.
Clinical characteristics and demographics of the study population.
| n | % |
|---|---|
| 50 | |
| 13 | 26 |
| 25 | 50 |
| 12 | 24 |
| 10 | 20% |
| 40 | 80% |
| 9 | 18% |
| 34 | 68% |
| 7 | 14% |
| 24 | 48% |
| 8 | 16% |
| 11 | 22% |
| 2 | 4% |
| 2 | 4% |
| 3 | 6% |
| 1 | 2% |
| 19 | 38% |
| 30 | 60% |
| 14 | 28% |
| 11 | 22% |
| 20 | 40% |
| 5 | 10% |
| 39 | 78% |
| 6 | 12% |
| 5 | 10% |
| 46 | 92% |
| 4 | 8% |
| 41 | 82% |
| 9 | 18% |
| 36 | 72% |
| 5 | 10% |
| 5 | 10% |
| 4 | 8% |
| 22 | 44% |
| 28 | 56% |
| 3 | 6% |
| 10 | 20% |
| 8 | 16% |
| 9 | 18% |
| 10 | 20% |
| 10 | 20% |
| 10 | 20% |
| 38 | 76% |
| 2 | 4% |
| 38 | 76% |
| 12 | 24% |
| 44 | 88% |
Radiation-associated toxicities
The five-day neaodjuvant RT regimen was well-tolerated without grade 3 or higher acute or toxicities. The most severe radiation dermatitis was grade 2 and occurred in four patients (8%); other grade 2 toxicities were pain flare (n=3, 6%) and nausea (n=1, 2%).
Radiation-associated toxicities (fibrosis, joint stiffness or lymphedema) as measured by RTOG/EORTC criteria are summarized in Table 2. No grade 3 or higher toxicities were observed after 29 months median follow-up (minimum 17 months). Overall, 7 of 44 evaluable patients (16%) developed at least one grade 2 radiation-associated toxicity, which met the primary endpoint. Grade 2 fibrosis (11%) and joint stiffness (11%) were more frequent than grade 2 lymphedema (4%). Of evaluable patients, 34 patients had minimum two years follow-up; five (14.7%) developed grade ≥2 fibrosis, lymphedema and/or joint stiffness. We observed a non-significant trend toward increased grade ≥2 radiation-associated toxicities in patients with tumors larger than the median size of 6.5 cm (p = 0.101, χ2 test), We did not observe any association between RT modality (IMRT, 3D, or electron) and toxicities, but the limited number of patients treated with 3D-conformal and electron RT limits this comparison (data not shown).
Table 2.
Late Toxicities of Five-Day Preoperative Radiation Therapy
| Fibrosis | |||
| Number of Patients | G1 | G2 | G3 |
| 11 (24%) | 5 (11%) | 0 (0%) | |
| Joint Stiffness | |||
| G1 | G2 | G3 | |
| 5 (11%) | 5 (11%) | 0 (0%) | |
| Lymphedema | |||
| G1 | G2 | G3 | |
| 2 (4%) | 2 (4%) | 0 (0%) | |
We also examined patient and physician-reported outcomes using TESS and MSTS score surveys, respectively, at baseline, 12, 18 and 24 months. Baseline and at least one evaluable follow-up data point were available in 34 of 50 patients. We did not observe a significant decline in functional outcome at 12, 18 or 24 months using either survey (Supplementary Figure 2A–B).
Wound Complications
Major wound complications were observed in 16 of 50 patients (32%). This rate is on par with rates of major wound complications observed in prospective studies of neaodjuvant RT (35% in the neoadjuvant RT arm of the NCIC Phase 3 study; 30.5% in multi-institutional phase 2 study of image-guided IMRT, and 36.6% in RTOG 0630) as well as retrospective analyses (Figure 1A)1–6. By CTCAE criteria, 12 patients (24%) experienced grade 3 or higher wound complication or wound dehiscence, including three patients who required a reconstruction flap (grade 4 complication). In the 16 patients with major wound complications, 14 have achieved wound closure at a median time to closure of 6.4 months (Figure 1B). There were more wound complications in patients with lower extremity tumors (p = 0.01; Figure 1C, Supplementary Table S2 and S3), including five out of nine patients with adductor compartment involvement. Wound complications were not associated with smoking history, time interval from radiation to surgery, tumor depth, tumor size or either of two parameters for radiation dose to the skin (Supplementary Figure S3A–D). The low rate of diabetes (n=4) in our study population precluded meaningful statistical analysis. We did not find an association of prior R1/R2 surgery on the incidence of major wound complications (p>0.99, Fisher’s exact test).
Figure 1.
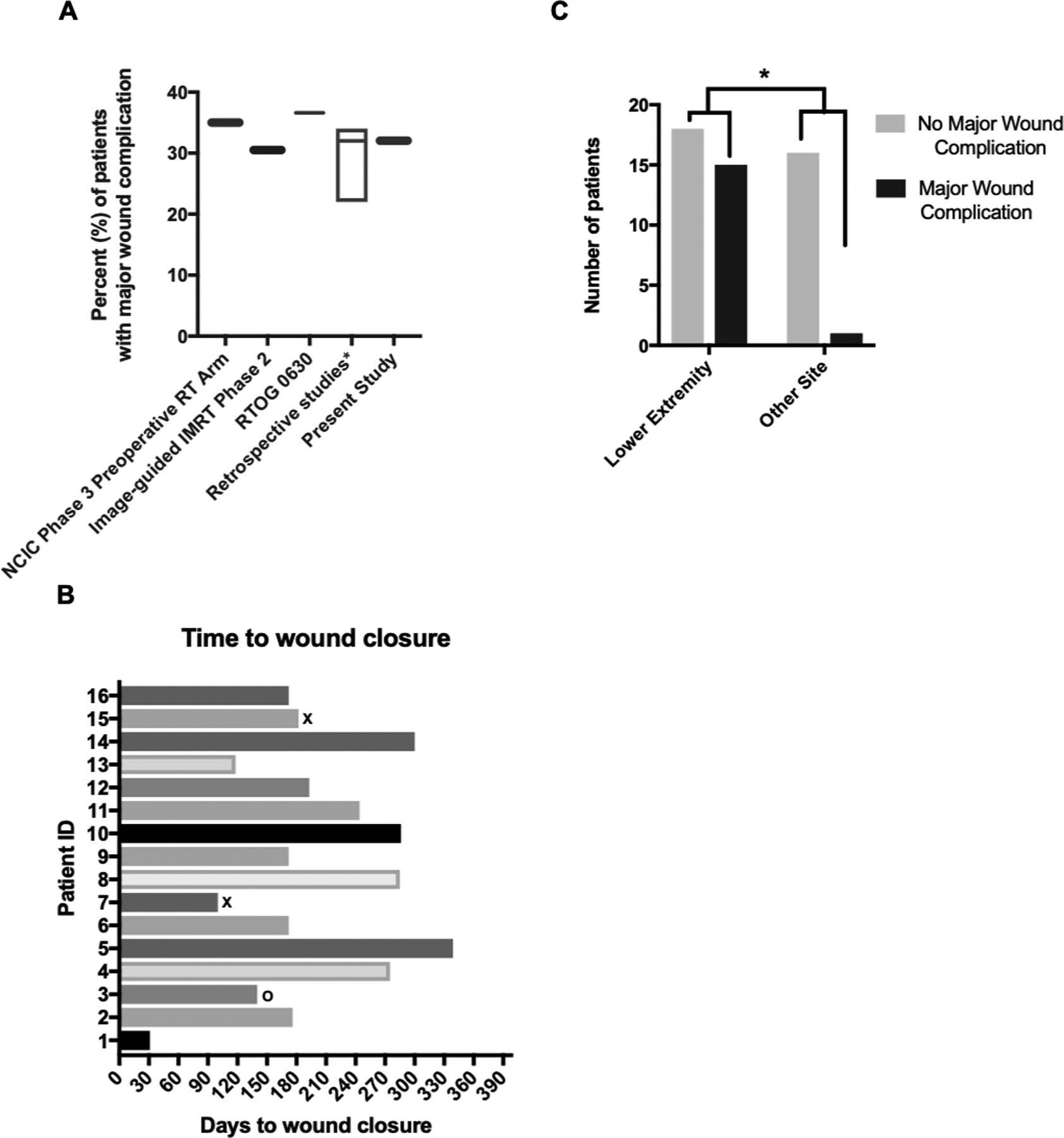
Characteristics of major wound complications. (A) The rate of major wound complications in the present study, alongside major wound complication rates from prospective studies (preoperative RT arm of the NCIC Randomized Phase 3 Study; image-guided IMRT phase 2 study of preoperative RT; and RTOG 0630)1–3 and modern retrospective studies4–6. (B) Time to wound closure in days. Patients who died (x) or underwent amputation (o) prior to wound closure are labeled. (C) Frequency of wound complications according to lower extremity tumor location compared to other sites (upper extremity and trunk).
Association of germline biomarkers with wound complications
Given the paucity of clinical factors that predict for wound complications, we hypothesized that inherent patient radiosensitivity may contribute to the risk of wound complications after neaodjuvant RT. In exploratory analysis, among lower extremity tumor site and a panel of 116 annotated SNPs in miRNA binding sites, tumor site and 19 SNPs were identified as the top 20 predictors for major wound complication rate (Supplementary Table S4). The prediction performance for four proposed classifiers (classification tree, random forest, boosted tree, and LASSO-LR) using these 20 predictors jointly were fairly similar (Table 3), with random forests performing the best, with an accuracy of 0.855, a specificity of 0.792, sensitivity of 0.917, AUC of 0.952 and F1 of 0.868.
Table 3.
Performance measures for prediction of major wound toxicity using germline biomarker and lower extremity tumor site.
| acc | specificity (TNR) | sensitivity (TPR) |
npv | ppv | AUC | F1 | |
|---|---|---|---|---|---|---|---|
| Classification Tree | 0.692 | 0.650 | 0.733 | 0.750 | 0.757 | 0.757 | 0.696 |
| Random Forest | 0.855 | 0.792 | 0.917 | 0.922 | 0.850 | 0.952 | 0.868 |
| Boosted Tree | 0.792 | 0.667 | 0.917 | 0.875 | 0.770 | 0.798 | 0.827 |
| LASSO-LR | 0.780 | 0.667 | 0.892 | 0.917 | 0.767 | 0.839 | 0.790 |
Early Oncologic Outcomes
Of the 50 patients who underwent surgery, a minimium of 2 years follow-up is available for 38 patients, of whom 35 are evaluable for local control (three patients have died). To date, two of these evaluable patients with minimum 2 years follow-up developed a local recurrence after surgery (5.7%); both local recurrences occurred within the first year of study enrollment. Ten of 47 evaluable patients (21.2%) have developed metastatic disease. There was a preponderance of high-grade tumors among patients who developed metastatic disease (80% versus 54%, p = 0.16), but no difference in size, tumor depth, or re-excision status (data not shown). Forty-two of 50 patients (84%) are alive, including six patients with metastatic disease. Of eight deaths, four patients have died due to causes unrelated to sarcoma or treatment (median age 81, range 70 – 90). Among 45 patients with evaluable pre- and post-treatment tumor, the average pathologic treatment effect was 44.2% (range 0 – 100%, standard deviation 31.6%) (Figure 2A)14.
Figure 2.
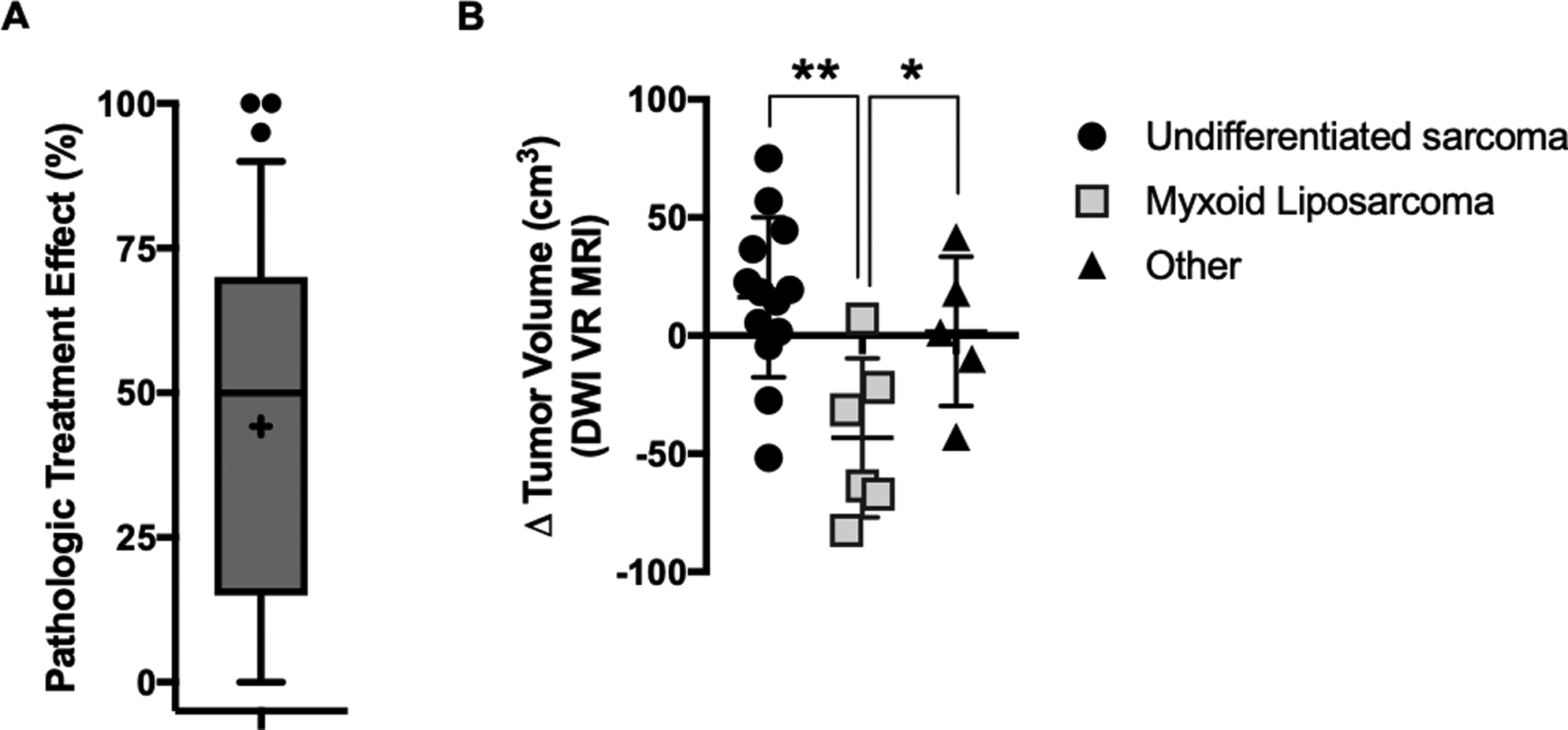
Early surrogates for clinical response to five-day neoadjuvant RT. (A) Treatment effect as measured by the percentage of necrosis and hyalinization in the surgical specimen relative to the biopsy specimen in patients treated on the five-day neoadjuvant RT protocol. Box plot represents 10th to 90th percentile, with mean (+), median, and outliers shown. (B) Pre-to-post treatment change in tumor volume by diffusion-weighted ViewRay MRI for n=25 patients with available data, according to histology (undifferentiated sarcoma, myxoid liposarcoma, and other). *, p<0.05; **, p<0.005 according to unpaired t test.
Twenty-five patients had matched pre- and post-treatment MRI available for analysis on the parallel imaging study (Supplementary Table S5). The median time between pre- and post-treatment scans was 20 days (range 16 – 35). Overall, there was no difference in DWI tumor volume between pre- and post-treatment scans, and there was no association between time interval between scans and change in DWI tumor volume. We observed a significant decrease in DWI tumor volume between pre- and post-treatment myxoid liposarcoma specimens (Δ = −43.2 cm3 +/− 13.7 cm3; p = 0.025), but not in non-myxoid liposarcoma tumors (Figure 2B).
Study-Associated Changes in Access and Utilization of RT
In the two-year period just prior to the initation of this study, 14 patients were treated with neaodjuvant RT at our institution. This value increased by three-fold (n=52) during the subsequent two-year study period (Figure 3A). In the two-year period just prior to the initiation of this study, patients treated with neaodjuvant RT lived at a median distance of 11 miles from our facility, and there were no patients who traveled over 100 miles to receive treatment. During the study period, the size of the catchment area increased; enrolled study patients traveled a median 56 miles from their primary residence to receive treatment (p<0.0001; Figure 3B) and 40% of patients traveled over 100 miles to receive treatment.
Figure 3.
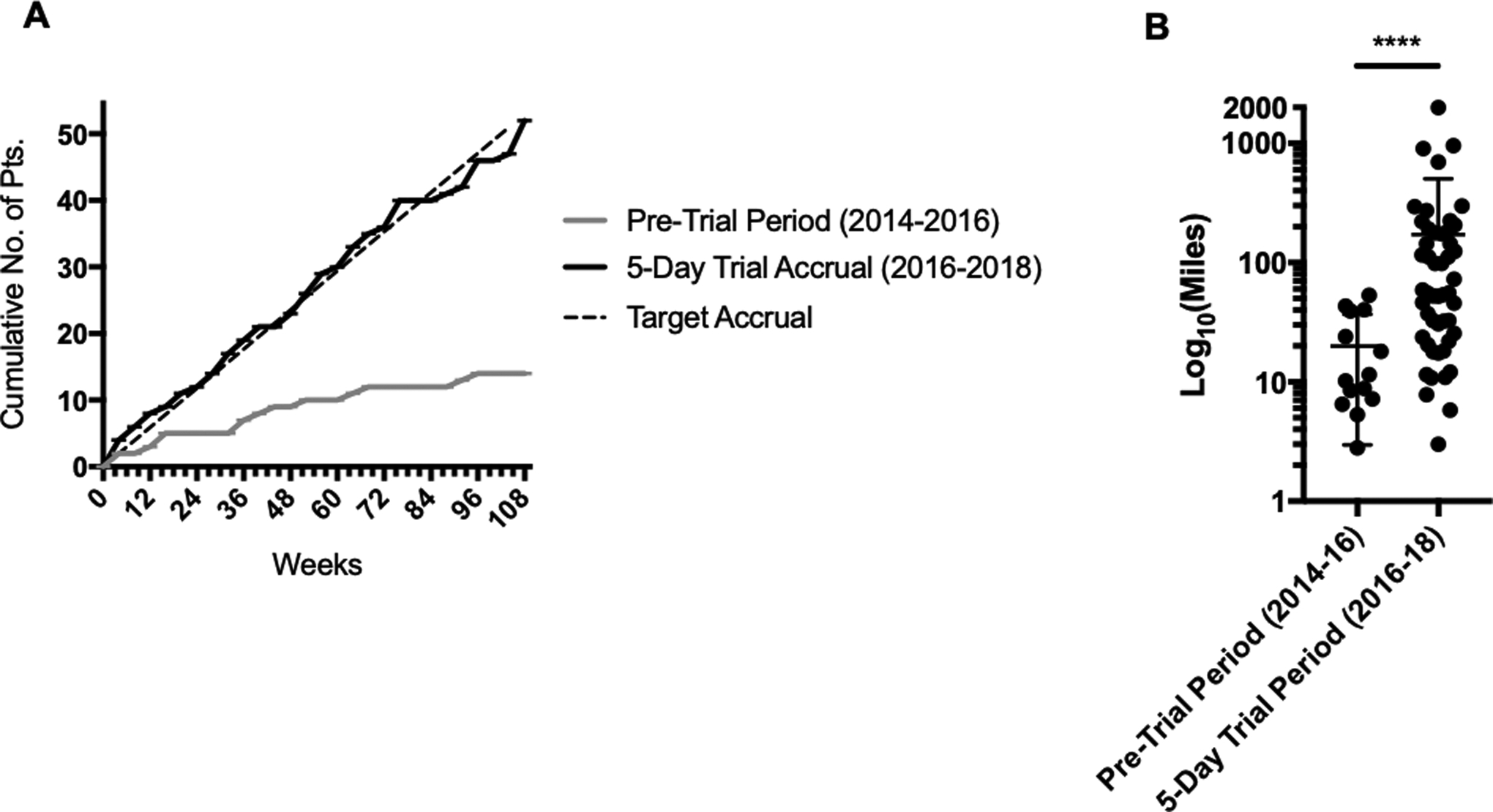
Impact of 5-day protocol on utilization of neoadjuvant RT at a high volume sarcoma center. (A) Cumulative accrual of patients to the phase 2 prospective study of five-day neoadjuvant RT, shown alongside target accrual rate and accrual of patients treated with standard 5-week neoadjuvant RT alone during the 2-year period preceding study initiation. (B) Distance traveled to our high volume sarcoma center for neoadjuvant RT by patients enrolled on the five-day phase 2 study and by patients in the 2-year period preceding study initation. ****, p<0.0001 (Mann-Whitney test).
DISCUSSION
STS is a rare malignancy and treatment at high-volume sarcoma centers has been associated with improved outcomes30–32. Conventional five-week RT is known to have poor utilization among patients traveling a long distance33,34 and is a barrier to treatment at high-volume sarcoma centers. Shorter RT regimens are not only preferred by patients35, but they reduce travel burden and increase the access to care at high-volume centers. To date, limited data exist on the morbidity of shorter, condensed neoadjuvant RT for the treatment of STS of the extremities and trunk. In our single-institution phase 2 study, five-day neoadjuvant RT to a total of 30 Gy was well-tolerated.
Shorter, condensed RT regimens with higher daily dose historically have not been used due to concerns about toxicities. The rate of grade ≥2 radiation-associated toxicity (fibrosis, joint stiffness, or lymphedema) after median follow-up of two years was tolerable (16%). However, while these rates are favorable compared to the CAN-NCIC-SR2 study1 against which our study was powered (16% versus 37%), and comparable to a more recent multi-institutional phase 2 study using modern image-guided RT3 that used 5-week neoadjuvant RT, longer follow-up is needed for a more robust comparison.
Another concern with RT dose intensification in the neoadjuvant setting was the rate of wound complications, which is the primary drawback of neaodjuvant RT for STS1. The rate of major wound complications in our study (32%) is consistent with results from previous prospective and retrospective studies that used conventional 5-week neaodjuvant RT (22 to 37%)1,3,4. The pattern of wound complications was also consistent with what is observed using 5-week neaodjuvant RT, with a propensity for complications in the lower extremity3. However, the median duration prior to wound closure was prolonged compared to a retrospective study of patients receiving conventionally fractionated RT36.
Given the complex and multifactorial nature of wound complications37, we examined whether specific clinical or dosimetric predictors could better identify patients at greatest risk. While clinical or dosimetric predictors other than lower extremity tumor location were not associated with wound toxicity, an exploratory analysis of germline SNPs in miRNA binding sites suggests a role for patient-intrinsic biology as a factor in the development of wound complications after neaodjuvant RT. There are indeed germline differences in radiosensitivity of normal tissues between individuals38–47. The majority of existing evidence on this topic centers on late skin and tissue response to radiotherapy of the breast. Our exploratory analysis identified a set of 19 germline alterations in microRNA binding sites in genes with roles in immune and DNA damage response that, in combination with lower extremity tumor location, are associated with major wound complications. These data are limited by the sample size of our phase 2 study, and validation of this set is necessary and ongoing. Nonetheless, these data highlight the potential for using patient germline features to stratify the risk of major wound complications prior to treatment. For these at-risk patients, more aggressive dosimetric constraints, consideration of adjuvant radiotherapy, alternative surgical approacheas or changes in post-surgical wound care may be warranted.
We observed both an increase in the number of patients treated with neoadjuvant RT and the distance traveled by patients to our high-volume center that coincided with study initiation. These results are consistent with prior studies demonstrating that shorter RT regimens are preferred by patients35 and suggest that five-day neoadjuvant RT would increase the utilization of neaodjuvant RT and access to care at high-volume sarcoma centers.
While local control results have yet to mature, early results are promising with two (5%) local failures among 35 evaluable patients with at least 2 years follow-up. The pathologic treatment effect observed in our study may serve as an early indicator of the anti-tumor efficacy of this regimen. Because the time interval from treatment initiation to surgery in our study is shorter, we anticipated pathologic response rates would be slightly lower than results from studies using standard 5-week neoadjuvant RT22,48 or studies using neoadjuvant chemoradiation49. As a secondary early indicator of clinical efficacy, we evaluated longitudinal diffusion-weighted MRI imaging of a subset of 25 patients. In the brief period between radiation and surgery (median 28 days), a brisk decrease in DWI tumor volume was noted in patients with myxoid liposarcoma, a histologic subtype characterized by inherent radiosensitivity, supporting the anti-tumor efficacy of the five-day radiation dose and fractionation.
Although we routinely incorporate neoadjuvant systemic therapy for high risk extremity and trunk STS, systemic therapy is not always recommended by our multidisciplinary conference due to age, comorbidities, clinicopathologic characteristics, and patient preference. The data presented here are not generalizable to patients with planned neoadjuvant or adjuvant chemotherapy. We are currently accruing to an expansion cohort of this phase 2 study to evaluate the safety of a five-day neoadjuvant RT regimen in combination with systemic therapy.
In conclusion, our results demonstrate that five-day neoadjuvant RT in extremity and trunk STS shows a favorable radiation toxicity profile at a median follow-up of 29 months and an acceptable rate of major wound complications. Importantly, we found a statisticaly significant increase in the number of patients treated with this short course of neoadjuvant RT at our high volume sarcoma treatment center, which suggests this protocol could improve neoadjuvant RT use and access to care of STS at high-volume sarcoma centers. Early local control, pathologic treatment effect and imaging outcomes support the bioactivity of this dose and fractionation scheme, though longer follow-up is needed. Finally, we identified a putative germline biomarker profile for major wound complications; further validation of this profile may guide safer utilization of neoadjuvant RT. In summary, five-day neoadjuvant RT is a safe, effective, and accessible alternative for patients with localized STS of the extremity and trunk that warrants evaluation in a larger multi-institutional study.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
A five-day neoadjuvant radiation therapy (RT) regimen for primary soft tissue sarcoma (STS) of the extremity and trunk demonstrates a toxicity profile similar to modern studies using conventional 5-week neaodjuvant RT. This less-burdensome five-day course of neoadjuvant RT increased access to care of STS at our high-volume sarcoma center, while early local control, pathologic and imaging outcomes support the bioactivity of this dose and fractionation scheme. We identified a putative germline biomarker profile for major wound complications; further validation of this profile may guide safer utilization of neoadjuvant RT. Together, these results support the evaluation of the five-day neoadjuvant RT regimen and its associated germline toxicity biomarker in a larger multi-institutional study for patients with primary soft tissue sarcoma (STS) of the extremity and trunk.
ACKNOWLEDGMENTS
The authors would like to thank the patients involved in this study for their critical participation, as well as the dosimetrists, medical physicists, nurses and radiation therapists for their outstanding patient care.
FINANCIAL SUPPORT: This study was funded by the Department of Radiation Oncology at UCLA. A.K. was supported by the following grants relevant to this study: UCLA CTSI KL2 Award (A.K.), Sarcoma Alliance for Research Through Collaboration (SARC) Career Enhancement Program (A.K.), Radiological Society for North America (RSNA) (A.K.), and Tower Cancer Research Foundation (A.K.).
Footnotes
DISCLOSURE OF POTENTIAL CONFLICTS OF INTEREST: J.W. co-founded MiraDx, a company that has created intellectual property regarding microRNA variants. F.C.E. serves on the scientific advisory board of Certis Oncology.
PRIOR PRESENTATIONS: Early results were presented at the annual Connective Tissue Oncology Society Meeting in 2017 (Maui, HI).
REFERENCES
- 1.O’Sullivan B, Davis AM, Turcotte R, et al. : Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet (London, England) 359:2235–41, 2002 [DOI] [PubMed] [Google Scholar]
- 2.O’Sullivan B, Griffin AM, Dickie CI, et al. : Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer 119:1878–84, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Wang D, Zhang Q, Eisenberg BL, et al. : Significant Reduction of Late Toxicities in Patients With Extremity Sarcoma Treated With Image-Guided Radiation Therapy to a Reduced Target Volume: Results of Radiation Therapy Oncology Group RTOG-0630 Trial. J Clin Oncol 33:2231–8, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.LeBrun DG, Guttmann DM, Shabason JE, et al. : Predictors of Wound Complications following Radiation and Surgical Resection of Soft Tissue Sarcomas. Sarcoma 2017:1–7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cannon CP, Ballo MT, Zagars GK, et al. : Complications of combined modality treatment of primary lower extremity soft-tissue sarcomas. Cancer 107:2455–61, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Tseng JF, Ballo MT, Langstein HN, et al. : The effect of preoperative radiotherapy and reconstructive surgery on wound complications after resection of extremity soft-tissue sarcomas. Ann Surg Oncol 13:1209–15, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Yang JC, Chang AE, Baker AR, et al. : Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. Journal of Clinical Oncology 16:197–203, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Gingrich AA, Bateni SB, Monjazeb AM, et al. : Neoadjuvant Radiotherapy is Associated with R0 Resection and Improved Survival for Patients with Extremity Soft Tissue Sarcoma Undergoing Surgery: A National Cancer Database Analysis. Ann Surg Oncol 24:3252–3263, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Venigalla S, Carmona R, VanderWalde N, et al. : Disparities in Perioperative Radiation Therapy Use in Elderly Patients With Soft-Tissue Sarcoma. Int J Radiat Oncol Biol Phys 102:155–165, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Folkert MR, Singer S, Brennan MF, et al. : Comparison of local recurrence with conventional and intensity-modulated radiation therapy for primary soft-tissue sarcomas of the extremity. J Clin Oncol 32:3236–41, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thames HD, Suit HD: Tumor radioresponsiveness versus fractionation sensitivity. International journal of radiation oncology, biology, physics 12:687–91, 1986 [DOI] [PubMed] [Google Scholar]
- 12.Livi L, Meattini I, Marrazzo L, et al. : Accelerated partial breast irradiation using intensity-modulated radiotherapy versus whole breast irradiation: 5-year survival analysis of a phase 3 randomised controlled trial. Eur J Cancer 51:451–63, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Erlandsson J, Holm T, Pettersson D, et al. : Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 18:336–346, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Pennington JD, Eilber FC, Eilber FR, et al. : Long-term Outcomes With Ifosfamide-based Hypofractionated Preoperative Chemoradiotherapy for Extremity Soft Tissue Sarcomas. American journal of clinical oncology:1, 2018. [DOI] [PubMed] [Google Scholar]
- 15.Mack LA, Crowe PJ, Yang JL, et al. : Preoperative chemoradiotherapy (modified Eilber protocol) provides maximum local control and minimal morbidity in patients with soft tissue sarcoma. Ann Surg Oncol 12:646–53, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Kosela-Paterczyk H, Szacht M, Morysinski T, et al. : Preoperative hypofractionated radiotherapy in the treatment of localized soft tissue sarcomas. Eur J Surg Oncol 40:1641–7, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Alektiar KM, Brennan MF, Singer S: Local control comparison of adjuvant brachytherapy to intensity-modulated radiotherapy in primary high-grade sarcoma of the extremity. Cancer 117:3229–34, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Alektiar KM, Brennan MF, Healey JH, et al. : Impact of intensity-modulated radiation therapy on local control in primary soft-tissue sarcoma of the extremity. J Clin Oncol 26:3440–4, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Haas RLM, Miah AB, LePechoux C, et al. : Preoperative radiotherapy for extremity soft tissue sarcoma; Past, present and future perspectives on dose fractionation regimens and combined modality strategies. Radiotherapy and Oncology 119:14–21, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis AM, Wright JG, Williams JI, et al. : Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 5:508–16, 1996 [DOI] [PubMed] [Google Scholar]
- 21.Enneking WF, Dunham W, Gebhardt MC, et al. : A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res:241–6, 1993 [PubMed] [Google Scholar]
- 22.Schaefer I-M, Hornick JL, Barysauskas CM, et al. : Histologic Appearance After Preoperative Radiation Therapy for Soft Tissue Sarcoma: Assessment of the European Organization for Research and Treatment of Cancer–Soft Tissue and Bone Sarcoma Group Response Score. International Journal of Radiation Oncology*Biology*Physics 98:375–383, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Chen X, Paranjape T, Stahlhut C, et al. : Targeted resequencing of the microRNAome and 3’UTRome reveals functional germline DNA variants with altered prevalence in epithelial ovarian cancer. Oncogene 34:2125–37, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Breiman L, Friedman J, Olshen R, et al. : Classification and Regression Trees. Boca Raton, FL, CRC Press, 2017 [Google Scholar]
- 25.Breiman L: Random Forests. Machine Learning 45:5–32, 2001 [Google Scholar]
- 26.Chen T, Guestrin C: XGBoost: A Scalable Tree Boosting System, KDD. San Francisco, CA, 2016 [Google Scholar]
- 27.Tibshirani R: Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society Series B (Methodological):267–88, 1996 [Google Scholar]
- 28.R Development Core Team: Stats package (power.prop.test() function) in R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2018 [Google Scholar]
- 29.Kahle D, Wickham H, Jackson S, et al. : Package ‘ggmap’, (ed 3.0.0), 2019
- 30.Venigalla S, Nead KT, Sebro R, et al. : Association Between Treatment at High-Volume Facilities and Improved Overall Survival in Soft Tissue Sarcomas. International Journal of Radiation Oncology*Biology*Physics 100:1004–1015, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abarca T, Gao Y, Monga V, et al. : Improved survival for extremity soft tissue sarcoma treated in high-volume facilities. J Surg Oncol 117:1479–1486, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gutierrez JC, Perez EA, Moffat FL, et al. : Should soft tissue sarcomas be treated at high-volume centers? An analysis of 4205 patients. Ann Surg 245:952–8, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroen AT, Brenin DR, Kelly MD, et al. : Impact of Patient Distance to Radiation Therapy on Mastectomy Use in Early-Stage Breast Cancer Patients. Journal of Clinical Oncology 23:7074–7080, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Athas WF, Adams-Cameron M, Hunt WC, et al. : Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst 92:269–71, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Hoopes DJ, Kaziska D, Chapin P, et al. : Patient preferences and physician practice patterns regarding breast radiotherapy. Int J Radiat Oncol Biol Phys 82:674–81, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Lansu J, Groenewegen J, van Coevorden F, et al. : Time dependent dynamics of wound complications after preoperative radiotherapy in Extremity Soft Tissue Sarcomas. Eur J Surg Oncol 45:684–690, 2019 [DOI] [PubMed] [Google Scholar]
- 37.Slump J, Bastiaannet E, Halka A, et al. : Risk factors for postoperative wound complications after extremity soft tissue sarcoma resection: A systematic review and meta-analyses. J Plast Reconstr Aesthet Surg 72:1449–1464, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Grossberg AJ, Lei X, Xu T, et al. : Association of Transforming Growth Factor beta Polymorphism C-509T With Radiation-Induced Fibrosis Among Patients With Early-Stage Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 4:1751–1757, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.West C, Rosenstein BS: Establishment of a radiogenomics consortium. Radiother Oncol 94:117–8, 2010 [DOI] [PubMed] [Google Scholar]
- 40.Chang-Claude J, Ambrosone CB, Lilla C, et al. : Genetic polymorphisms in DNA repair and damage response genes and late normal tissue complications of radiotherapy for breast cancer. Br J Cancer 100:1680–6, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Isomura M, Oya N, Tachiiri S, et al. : IL12RB2 and ABCA1 genes are associated with susceptibility to radiation dermatitis. Clin Cancer Res 14:6683–9, 2008 [DOI] [PubMed] [Google Scholar]
- 42.Giotopoulos G, Symonds RP, Foweraker K, et al. : The late radiotherapy normal tissue injury phenotypes of telangiectasia, fibrosis and atrophy in breast cancer patients have distinct genotype-dependent causes. Br J Cancer 96:1001–7, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Damaraju S, Murray D, Dufour J, et al. : Association of DNA repair and steroid metabolism gene polymorphisms with clinical late toxicity in patients treated with conformal radiotherapy for prostate cancer. Clin Cancer Res 12:2545–54, 2006 [DOI] [PubMed] [Google Scholar]
- 44.Andreassen CN, Alsner J, Overgaard M, et al. : Risk of radiation-induced subcutaneous fibrosis in relation to single nucleotide polymorphisms in TGFB1, SOD2, XRCC1, XRCC3, APEX and ATM--a study based on DNA from formalin fixed paraffin embedded tissue samples. Int J Radiat Biol 82:577–86, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Andreassen CN, Alsner J, Overgaard M, et al. : Prediction of normal tissue radiosensitivity from polymorphisms in candidate genes. Radiother Oncol 69:127–35, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Quarmby S, Fakhoury H, Levine E, et al. : Association of transforming growth factor beta-1 single nucleotide polymorphisms with radiation-induced damage to normal tissues in breast cancer patients. Int J Radiat Biol 79:137–43, 2003 [PubMed] [Google Scholar]
- 47.Angele S, Romestaing P, Moullan N, et al. : ATM haplotypes and cellular response to DNA damage: association with breast cancer risk and clinical radiosensitivity. Cancer Res 63:8717–25, 2003 [PubMed] [Google Scholar]
- 48.Macchia G, Gambacorta MA, Masciocchi C, et al. : Time to surgery and pathologic complete response after neoadjuvant chemoradiation in rectal cancer: A population study on 2094 patients. Clin Transl Radiat Oncol 4:8–14, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eilber FC, Rosen G, Eckardt J, et al. : Treatment-induced pathologic necrosis: a predictor of local recurrence and survival in patients receiving neoadjuvant therapy for high-grade extremity soft tissue sarcomas. J Clin Oncol 19:3203–9, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


