Figure 2.
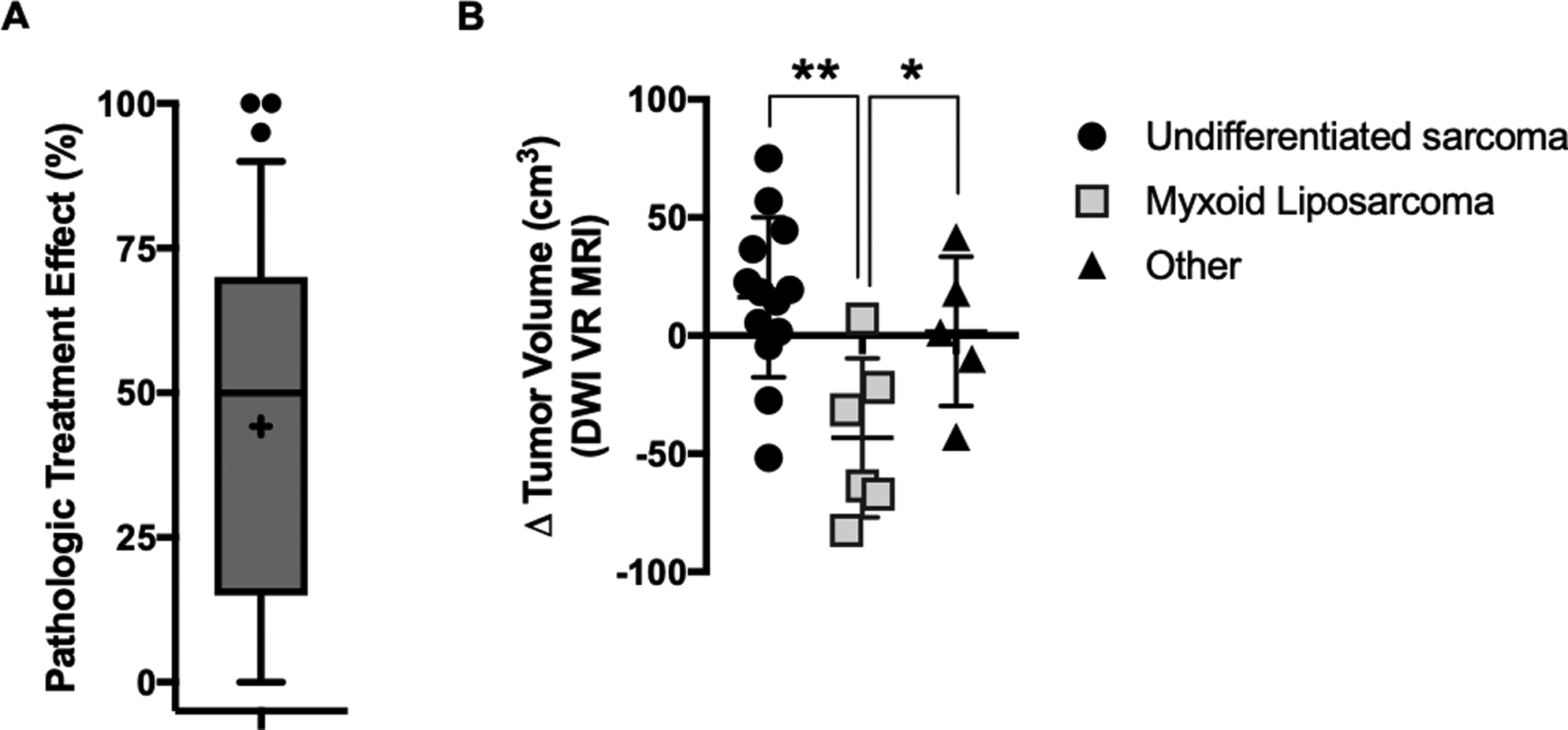
Early surrogates for clinical response to five-day neoadjuvant RT. (A) Treatment effect as measured by the percentage of necrosis and hyalinization in the surgical specimen relative to the biopsy specimen in patients treated on the five-day neoadjuvant RT protocol. Box plot represents 10th to 90th percentile, with mean (+), median, and outliers shown. (B) Pre-to-post treatment change in tumor volume by diffusion-weighted ViewRay MRI for n=25 patients with available data, according to histology (undifferentiated sarcoma, myxoid liposarcoma, and other). *, p<0.05; **, p<0.005 according to unpaired t test.
