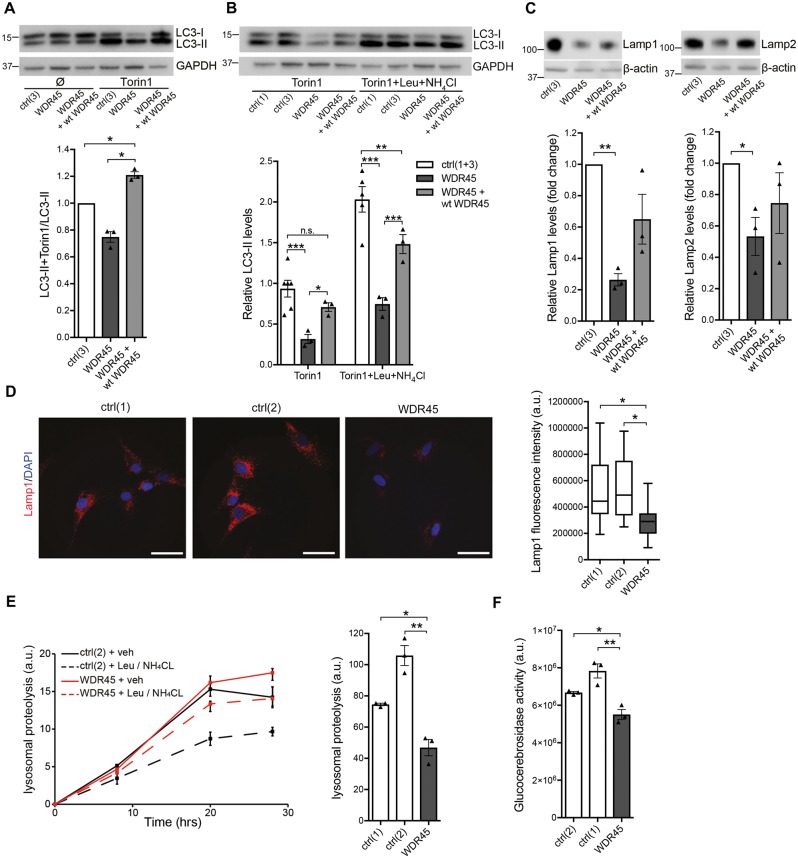
Figure 4.
Altered autophagy and lysosomal function in WDR45 mutant fibroblasts. (A) Western blot analysis from control (ctrl), WDR45 mutant, and WDR45 mutant fibroblasts overexpressing wild-type WDR45 using lentiviral vectors. Samples were collected from cells cultured under basal conditions and treated with the mTOR inhibitor torin 1. The western blot was probed against the autophagosome marker LC3-II (membrane-bound form), a cleavage product of LC3-I, and GAPDH (loading control). (B) Control and WDR45 mutant cells were treated with torin 1 alone and torin 1 plus the lysosomal inhibitors leupeptin (Leu) and ammonium chloride (NH4Cl). The western blot was probed against LC3-II and GAPDH (loading control). (C) Levels of the lysosomal proteins LAMP1 and LAMP2 were detected by western blotting. GAPDH was used as loading control. Statistical significance was analysed by one-way ANOVA (LAMP1) and selected unpaired t-test (LAMP2). (D) Immunofluorescence staining of control and WDR45 mutant fibroblasts against LAMP1 (red) and nuclear DAPI (blue). (E) Using radioactive pulse-chase experiments in living cells, lysosomal proteolysis rates were examined in mutant compared to control fibroblasts. To distinguish between proteolysis that occurs within the lysosomal compartment or other degradation pathways, the response to lysosomal inhibitors was measured. (F) Lysosomal glucocerebrosidase activity was measured in whole-cell lysates from WDR45 mutant and control fibroblasts.