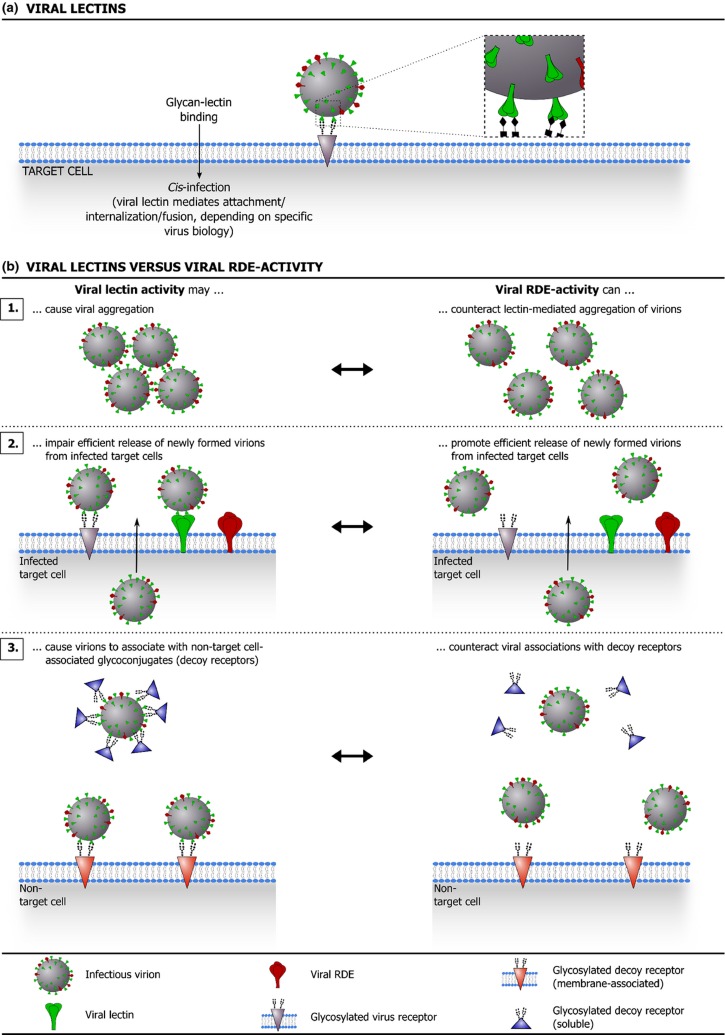
Figure 5.
(a) illustrates how viral lectins promote target cell infection. (b) shows how many viruses that employ viral lectins also benefit from a matching receptor-destroying enzyme (RDE) activity, which provides a counterweight against (high avidity) lectin activity. (a) Interaction of viral lectins with glycosylated receptors on a target cell promotes viral entry and infection (attachment/internalization/fusion, depending on specific virus biology). (b) Although they clearly benefit the virus, the use of (high avidity) viral lectins comes with a price. For instance, viral lectin activity can cause virions to aggregate (b.1) and can impair efficient release of newly formed virions from (glycosylated) infected cells (b.2). Moreover, binding of viral lectins to nontarget cell-associated glycoconjugates (decoy receptors) can prevent the virus from efficiently targeting susceptible host cells (b.3). Intriguingly, several lectin-carrying viruses are also equipped with an RDE that matches the specificity of the viral lectin and provides a counterweight against lectin-mediated glycan binding. In fact, for viruses equipped with both viral lectins and RDEs, a functional balance between these molecules appears to be an important determinant of the viral (replicative) fitness.