Figure 2. The KinB/AlgB pathway modulates Cas3 and Csy protein and RNA levels.
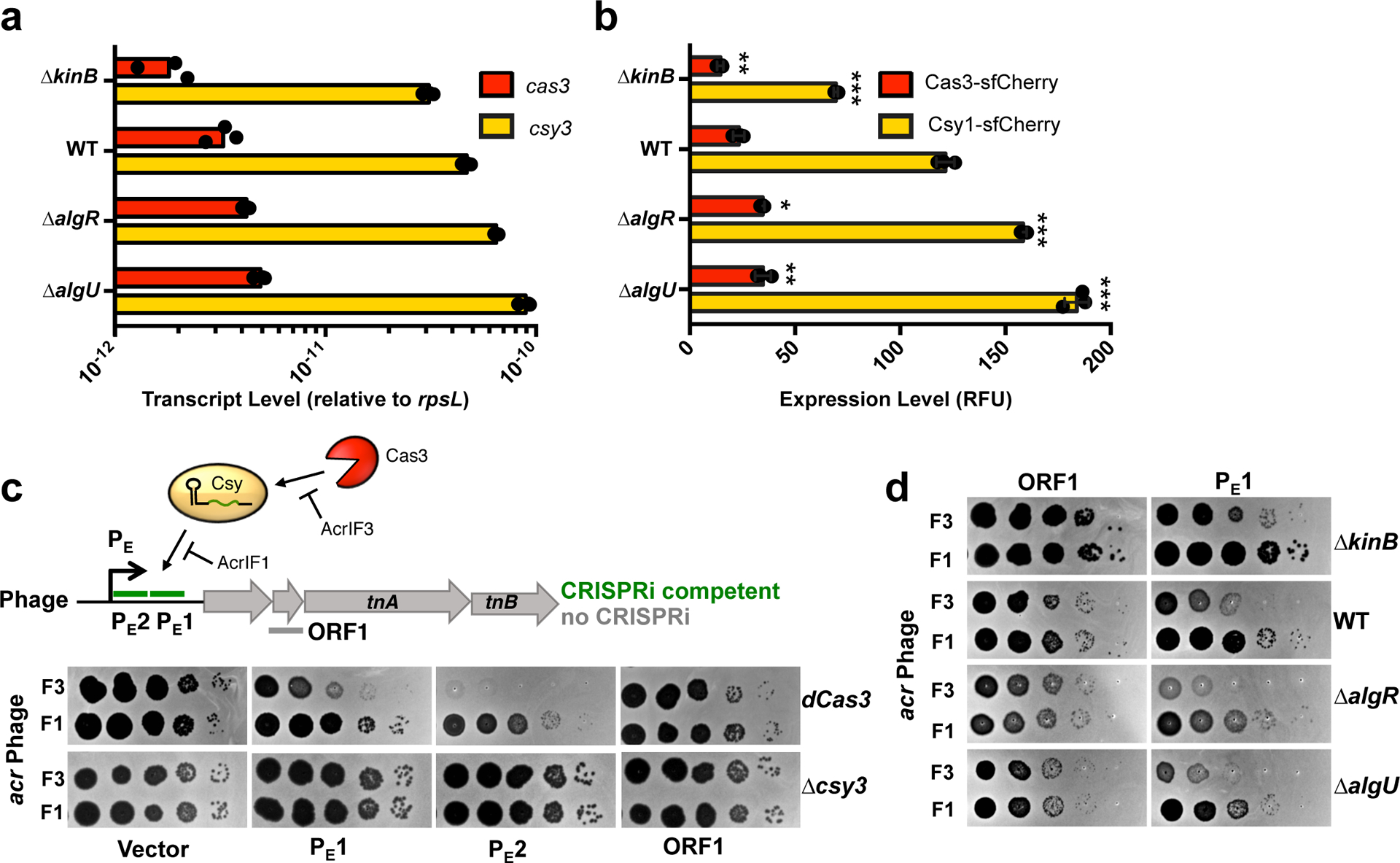
a. qRT-PCR measurements of transcript levels of cas3 (red) and csy3 (yellow) normalized to the housekeeping gene rpsL after 8 h of growth in liquid culture. Measurements are represented as the mean of 3 technical replicates. b. Measurement of the fluorescence levels of Cas3-sfCherry (red) or Csy1-sfCherry (yellow) reporter strains after 10 h of growth in liquid culture. Fluorescence measurements are represented as the mean of 3 biological replicates +/− SD. Mutants show altered Cas3-sfCherry levels (ΔkinB, P = 7.8 × 10−3 ΔalgR, P = 1.5 × 10−4 ΔalgU, P = 1.1 × 10−4) and Csy1-sfCherry levels relative to WT (ΔkinB, P = 3.3 × 10−5, ΔalgR, P = 1.5 × 10−4, ΔalgU, P = 1.1 × 10−4). Two-tailed unpaired Student’s T-test was used to calculate P values,*p < 0.05, **p < 0.01, ***p < 0.001. c. Spot titration of F3 (DMS3macrIF3) or F1 (DMS3macrIF1) on dCas3 (dead Cas3) or Δcsy3 (active Cas3, no Csy complex) strains. Phages are targeted by natural spacer CR2_sp1, as well as crRNAs designed to target DMS3m genome in positions designated on ORF map. d. Spot titration of DMS3macrIF3 and DMS3macrIF1 phages on WT PA14 or deletion mutants expressing the indicated crRNA. Plaquing experiments were replicated 3 times and consistent results seen.
