Figure 1.
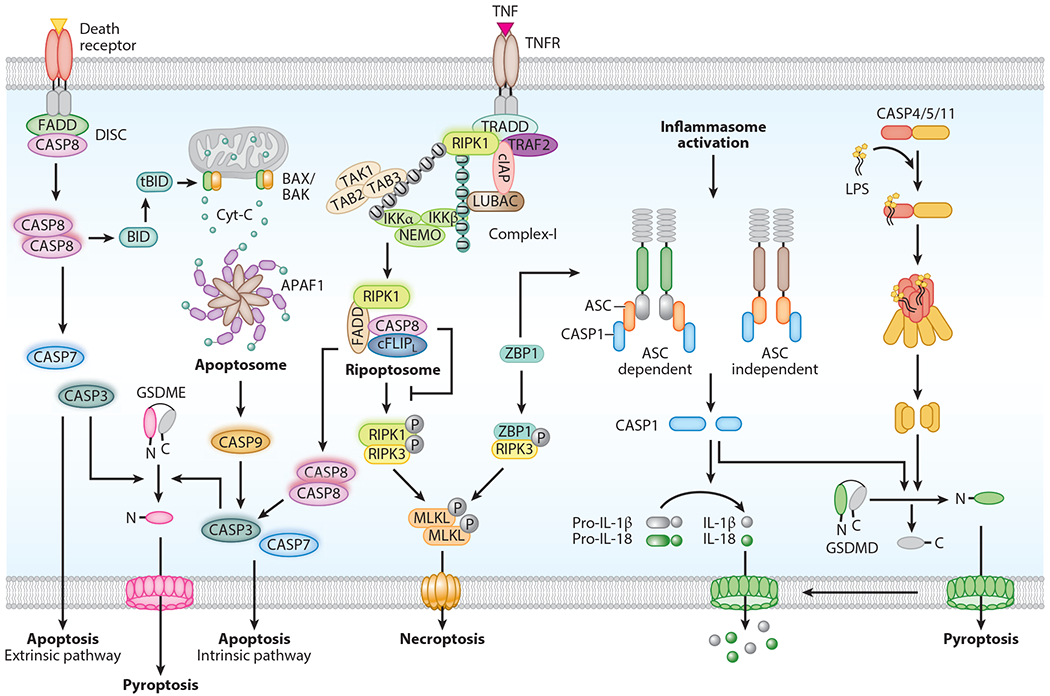
Mechanism of caspase activation and execution of cell death. Caspases trigger activation of apoptosis, a noninflammatory form of cell death. In extrinsic apoptosis, death receptor activation facilitates recruitment of FADD and caspase-8 to form the death-inducing signaling complex (DISC). Activated caspase-8 in the DISC further processes executioner caspases (caspase-3, -7) to engage apoptosis. In intrinsic apoptosis, intracellular stress stimuli induce mitochondrial outer membrane permeabilization (MOMP). This leads to the release of cytochrome c into the cytosol. Binding of cytochrome c to APAF1 triggers assembly of the apoptosome complex, which facilitates activation of caspase-9. This in turn promotes activation of caspase-3 and -7 to execute apoptosis. Complex-I assembly is initiated by TNF binding to TNFR on the membrane. This promotes prosurvival signaling via receptor-interacting serine/threonine protein kinase 1 (RIPK1), TAK-TAB, and NEMO proteins. Loss of RIPK1 ubiquitination promotes formation of the intracellular ripoptosome complex. Inactivation of the catalytic activity of caspase-8 in the ripoptosome complex induces RIPK1-RIPK3 association and phosphorylation of MLKL to form pores on the membrane and engage necroptosis. ZBP1 also activates necroptosis via RIPK3. Activation of inflammatory caspases induces pyroptosis. Inflammasome assembly activates caspase-1 enzymatic function. ASC in the inflammasome complex recruits caspase-1. Some inflammasome-forming innate immune receptors recruit caspase-1 without the involvement of an ASC protein. ZBP1 activates the NLRP3 inflammasome in response to influenza infection. LPS binds to caspase-11 (or human caspase-4/-5) and triggers its activation through oligomerization and cleavage. Activation of caspase-1 and -11 proteolytically processes gasdermin D (GSDMD) to release the N-terminal domain, which forms pores on the membrane to further induce pyroptosis. Caspase-1 also cleaves pro-IL-1β and pro-IL-18 into IL-1β and IL-18, which are released through GSDMD pores. Caspase-3-mediated gasdermin E (GSDME) cleavage also drives pyroptosis.
