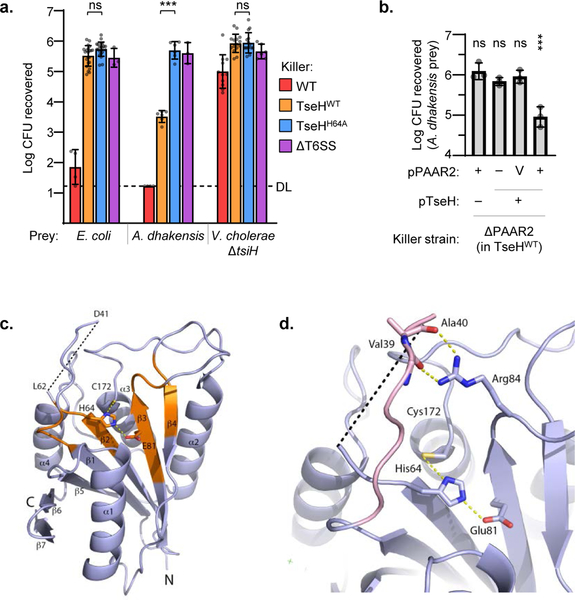
Figure 1: TseH is an NlpC/P60 peptidase delivered by PAAR2 that kills A. dhakensis but not E. coli or V. cholerae.
a. T6SS competition assay wherein E. coli, A. dhakensis , or V. cholerae ΔtsiH prey were subjected to killing by wild-type (WT) or catalytically inactive (H64A) TseH delivered from V52 with all other anti-bacterial effectors inactivated. One-way ANOVA with Sidak’s multiple comparisons test comparing each sample to the same prey killed by the TseHH64A strain; ***, p < 0.001; ns, not significant. DL, detection limit. b. Survival of A. dhakensis prey after killing by ΔPAAR2 (in TseHWT background) strain expressing plasmid-borne TseH and/or PAAR2. V, vector control; One-way ANOVA with Dunnett’s multiple comparisons test comparing to the sample expressing TseH and Vector control of PAAR2; ***, p < 0.001; ns, not significant. Graphs show the mean of at least three independent replicates and error bars show one standard deviation. Dots show individual replicates. WT, wild-type V52; TseHWT, V52 with all anti-bacterial effectors inactivated except TseH; TseHH64A, V52 with all anti-bacterial effectors inactivated including TseH. c. Crystal structure of TseH. Brown highlights conserved regions identified previously4. C172 was modified by a cacodylate ion in the crystal structure but is illustrated in stick format without the modification for illustration purpose. The area cleaved by thermolysin is depicted as a dotted line. d. Putative substrate binding pocket of TseH. The catalytic triad is shown as a stick model. The loop covering the active site (pink) is stabilized by the hydrogen bonding interaction (yellow dotted line) between Arg84 side chain and backbone carbonyls of Val39 and Ala40. Val39 side chain is not shown for illustration purpose.