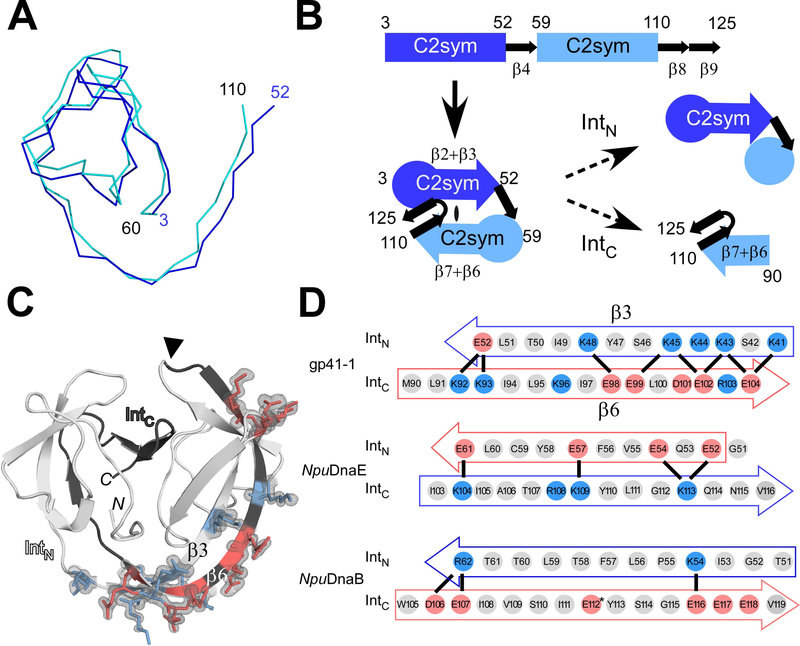
Fig. 3.
The modular architecture of the gp41–1 intein and the charge network. (A) An overlay of the backbone atoms of the two C2-symmetry-related units (residues 3–52 and 59–110) observed in the gp41–1 intein structure. (B) The arrangement of the C2-symmetry-related units and connections of the secondary structures. The natural split site of the gp41–1 intein locates within the second C2-symmetry-related part and at the front of strand β6. (C) The charged network found in the gp41–1 intein structure. The side-chains of the charged residues in the β3 and β6 strands are shown together with the electron density map. Residues with negative and positive charges are highlighted in red and blue, respectively. A filled triangle indicates the natural split site. N and C indicate the termini. (D) Comparison of the charged residues in the β3 and β6 strands between the gp41–1, NpuDnaE, and NpuDnaB inteins. Thick lines indicate possible favored charge interactions. An asterisk indicates Glu112 modeled as Val112 in the coordinate of the NpuDnaB mini-intein structure (PDB: 4o1r). (A, C) Figures were produced by PyMOL.