Abstract
Purpose
This study was designed to identify the pathogenic variants in three consanguineous families with congenital cataracts segregating as a recessive trait.
Methods
Consanguineous families with multiple individuals manifesting congenital cataracts were ascertained. All participating members underwent an ophthalmic examination. A small aliquot of the blood sample was collected from all participating individuals, and genomic DNAs were extracted. Homozygosity-based linkage analysis was performed using short tandem repeat (STR) markers. The haplotypes were constructed with alleles of the STR markers, and the two-point logarithm of odds (LOD) scores were calculated. The candidate gene was sequenced bidirectionally to identify the disease-causing mutations.
Results
Linkage analysis localized the disease interval to chromosome 3p in three families. Subsequently, bidirectional Sanger sequencing identified two novel mutations—a single base deletion resulting in a frameshift (c.3196delC; p.His1066IlefsTer10) mutation and a single base substitution resulting in a nonsense (c.4270C>T; p.Arg1424Ter) mutation—and a known missense (c.4127T>C, p.Leu1376Pro) mutation in FYCO1. All three mutations showed complete segregation with the disease phenotype and were absent in 96 ethnically matched control individuals.
Conclusions
We report two novel mutations and a previously reported mutation in FYCO1 in three large consanguineous families. Taken together, mutations in FYCO1 contribute nearly 15% to the total genetic load of autosomal recessive congenital cataracts in this cohort.
Introduction
A cataract is the manifestation of ocular lens opacification [1-3]. The principal function of the lens is to transmit light and focus it on the retina. Then the retina transforms the light into visual signals [4,5]. The transparency of the lens stems from the complete loss of organelles during the differentiation of lens fiber cells [6]. Cataracts are classified according to their morphology and the location of the opacity in the lens [7]. Congenital cataract (CC) is the primary cause of visual impairment in children worldwide [8]. The prevalence of isolated CC in industrialized countries is estimated at 1–6/10,000 live births [9-11], whereas these numbers are estimated to be 5–15/10,000 in developing countries [12]. Cataracts contribute nearly 39.1% of total blindness globally; however, the proportion is considerably higher (51.5%) in Pakistan. Congenital cataracts account for 23.0% of the total 54.7% visually handicapped children in Pakistan [13,14].
To date, 32 genes and loci have been implicated in non-syndromic autosomal recessive CC (arCC). Causative mutations in EPHA2 (Gene ID 1969, OMIM 176946), GJA8 (Gene ID 2703, OMIM 600897), FOXE3 (Gene ID 2301, OMIM 601094), FYCO1 (Gene ID 79443, OMIM 607182), GCNT2 (Gene ID 2651, OMIM 600429), AGK (Gene ID 55750, OMIM 610345), AKR1E2 (Gene ID 83592, OMIM 617451), RNLS (Gene ID 55328, OMIM 609360), DNMBP (Gene ID 23268, OMIM 611282), CRYAB (Gene ID 1410, OMIM 123590), MIP (Gene ID 4284, OMIM 154050), GJA3 (Gene ID 2700, OMIM 121015), HSF4 (Gene ID 3299, OMIM 602438), LONP1 (Gene ID 9361, OMIM 605490), WDR87 (Gene ID 83889), SIPA1L3 (Gene ID 23094, OMIM 616655), LIM2 (Gene ID 3982, OMIM, 154045), BFSP1 (Gene ID 631, OMIM 603307), BFSP2 (Gene ID 8419, OMIM 603212), CRYAA (Gene ID 1409, OMIM 123580), CRYBA1 (Gene ID 1411, OMIM 123610), LSS (Gene ID 4047, OMIM 600909), CRYBB3 (Gene ID 1417, OMIM 123630), CRYBB1 (Gene ID 1414, OMIM 600929), CRYBA4 (Gene ID 1413, OMIM 123631), TDRD7 (Gene ID 23424, OMIM 611258), and GALK1 (Gene ID 2584, OMIM 604313) have been implicated in CC [15-35]. In addition to genes, five loci (3q, 7q, 8p, 9q, and 19q) have been reported for CC [36-40]. Genetic mutations leading to impaired protein folding and solubility in lens fiber cells account for one-third of the total isolated CC cases [41-43]. Approximately 50% and 25% of the total mutations causing isolated CC have been reported in genes encoding crystallin and connexin proteins, respectively [44].
FYVE and coiled-coil domain containing 1 (FYCO1), an autophagy adaptor protein, interacts with microtubule-associated protein 1 light chain 3B (MAP1LC3B), phosphatidylinositol-3-phosphate (PI3P), and RAB7. We have previously shown that multiple loss-of-function mutations in FYCO1 result in arCC [17], and contribute to nearly 14% of the total genetic load of arCC in Pakistani families (16/116) [18]. In this study, we screened 13 other families and identified two novel and a previously reported mutation in FYCO1 bringing the total genetic contribution of mutations in FYCO1 responsible for arCC in this cohort to 15% (19/129).
Methods
Ascertainment of families and clinical evaluation
A large cohort of consanguineous Pakistani families (>200) with two or more affected individuals with congenital cataracts without any environmental or systemic involvement was recruited in a collaborative study to identify new disease-causing loci for congenital visual disorders. Institutional review boards (IRBs) of the National Centre of Excellence in Molecular Biology (Lahore, Pakistan), the National Eye Institute (Bethesda, MD), and the Johns Hopkins University (Baltimore, MD) granted approval for this study. Informed written consent adhering to the tenets of the Declaration of Helsinki was signed by each participating subject.
Thirteen families with non-syndromic arCC were selected for the present study. Detailed family and medical histories were compiled by reviewing available medical records and interviewing family members. Ophthalmic examination of all the participating subjects was conducted with slit-lamp microscopy at the Layton Rahmatulla Benevolent Trust Hospital (Lahore, Pakistan). Affected and unaffected members of each family donated about 10 ml of a blood sample which was collected in 50 ml Sterilin® Falcon tubes (BD Biosciences, San Jose, CA) that had 400 µl of 0.5 M EDTA. For long-term storage, blood samples were placed at -20 °C. Genomic DNAs were extracted from white blood cells using a organic method as described previously [23]. The concentration of the extracted DNA was estimated using a SmartSpec plus BIO-Rad Spectrophotometer (Bio-Rad, Hercules, CA).
Exclusion analysis
Short tandem repeat (STR) marker-based exclusion analysis was performed for 19 reported genes and loci previously associated with arCC. They included D1S402, D1S436, D1S2697, D1S1592, D1S2826, and D1S2864 for EPHA2; D1S496, D1S186, D1S432, D1S3721, D1S197, D1S2652, and D1S2890 for FOXE3; D1S2726, D1S252, D1S498, and D1S2635 for GJA8; D3S3527, D3S3685, D3S3582, D3S1767, D3S1581, and D3S1289 for FYCO1; D6S1034, D6S1653, and D6S429 for GCNT2; D7S2513, D7S661, and D7S636 for AJK; D11S2017, D11S1986, and D11S4111 for CRYAB; D16S3043, D16S3086, and D16S421 for HSF4; D17S1301 and D17S1839 for GALK1; D19S246, D19S589, and D19S254 for LIM2; D20S852, D20S112, D20S860, and D20S912 for BFSP1; D21S1411 and D21S1259 for CRYAA; D22S419, D22S1167, and D22S1144 for CRYBB1; D22S427, D22S686, D22S1167, D22S1144, and D22S689 for CRYBB3; D3S1565, D3S3715, and D3S3609 for chromosome 3q; D7S492, D7S657, D7S2430, D7S2482, D7S515, D7S692, and D7S2554 chromosome 7q; D8S550, D8S552, D8S1827, D8S549, and D8S1734 for chromosome 8p; D9S933, D9S167, D9S776, and D9S1790 for chromosome 9q; and D19S433, D19S416, and D19S220 for chromosome 19q loci.
PCRs with fluorescently labeled primer pairs were performed in a GeneAmp PCR System 2700 thermocycler (Applied Biosystems, Waltham, MA). Concisely, each reaction was completed in 5 μl reaction volume containing 50 ng genomic DNA as template, 0.15 μl of 10 mM dye-labeled primer pair, 0.5 μl of 10X PCR Buffer [100 mM Tris HCl (pH 8.5), 500 mM KCl, 15 mM MgCl2], 0.5 μl of 10 mM dNTP mix, and 0.2 μl of 5 U/μl Taq DNA polymerase. Initial denaturation was performed for 5 min at 95 °C, followed by 35 cycles of 30 s at 94 °C for denaturation, 45 s at 54 °C for annealing, 2 min at 65 °C for extension, and then 10 min at 72 °C for a final extension step. Amplified products from each DNA sample were pooled (up to 20) and mixed with an HD-400 size standards (Applied Biosystems) loading cocktail. The resulting amplicons were resolved in a 3730 DNA Analyzer (Applied Biosystems), and genotypes were assigned with ABI PRISM GeneMapper Software v4.0 (Applied Biosystems).
Linkage analysis
The FASTLINK version of MLINK from the LINKAGE Program Package (provided in the public domain by the Human Genome Mapping Project Resources Centre, Cambridge, UK) was used to perform two-point linkage analyses and to calculate the maximum logarithm of odds (LOD) scores (Zmax) [45,46]. Autosomal recessive CC was analyzed as a fully penetrant trait with 0.001 affected allele frequency. The order of the markers and the distances between them were obtained from the Marshfield database and the National Center for Biotechnology Information (NCBI, Bethesda, MD) chromosomes sequence maps. Allele frequencies were estimated from 96 unrelated and unaffected individuals from the Punjab province of Pakistan.
Sanger sequencing
Primer pairs (forward and reverse) for FYCO1 were designed using the Primer3 (Ver. 0.4.0). Amplifications were performed in a 25 µl mixture containing 50 ng of genomic DNA, 0.5 µl of each primer (4 µM), 2.5 μl of 10X PCR Buffer [100 mM Tris HCl (pH 8.5), 500 mM KCl, 15 mM MgCl2], 1.25 μl of 10 mM dNTP mix, and 1 μl of 5 U/μl Taq DNA polymerase. PCR amplification of exons covered an initial denaturation step for 5 min at 95 °C followed by a two-step procedure. The first touchdown step of ten cycles consisted of 30 s denaturation at 95 °C, followed by annealing at 68 °C for 30 s (annealing temperature decreased by 1 °C/cycle), and 1-min extension at 72 °C. The second step of 30 cycles consisted of 30 s denaturation at 95 °C, followed by annealing at 58 °C for 30 s (10 °C below the annealing temperature of the first step), 1-min extension at 72 °C, and then a final extension step of 10 min at 72 °C. Amplicons were analyzed on 1.5% agarose gel and purified with 95% ethanol precipitation. The PCR primers for each exon were used for bidirectional Sanger sequencing using BigDye Terminator ready reaction mix (Applied Biosystems) according to the manufacturer’s instructions. Sequencing products were precipitated (sodium acetate, EDTA, and ethanol), resuspended in 10 µl of formamide (Applied Biosystems), denatured for 5 min at 95 °C, and resolved on a 3730 DNA Analyzer (Applied Biosystems). Forward and reverse sequencing results were assembled with ABI PRISM® sequencing analysis software (Ver. 3.7) and analyzed with Sequencher software (Gene Codes Corporation, MI).
Prediction analysis
Evolutionary conservation of the mutated amino acid in FYCO1 orthologs was examined using the UCSC Genome Browser. The possible impact of amino acid substitution on the structure of the FYCO1 protein at the location of the missense mutation was examined with PolyPhen-2, Mutation Assessor, Mutation Taster, and SIFT.
Results
Three consanguineous families (PKCC193, PKCC202, and PKCC220) were recruited from the Punjab province of Pakistan. Pedigree drawings demonstrated an autosomal recessive mode of inheritance (Figure 1). Detailed medical history obtained after interviews with family members, and the patient’s available medical records confirmed that cataracts were observed in the first or second year after birth and segregated in an isolated fashion without any other ocular and non-ocular anomalies in affected individuals of three families (Table 1).
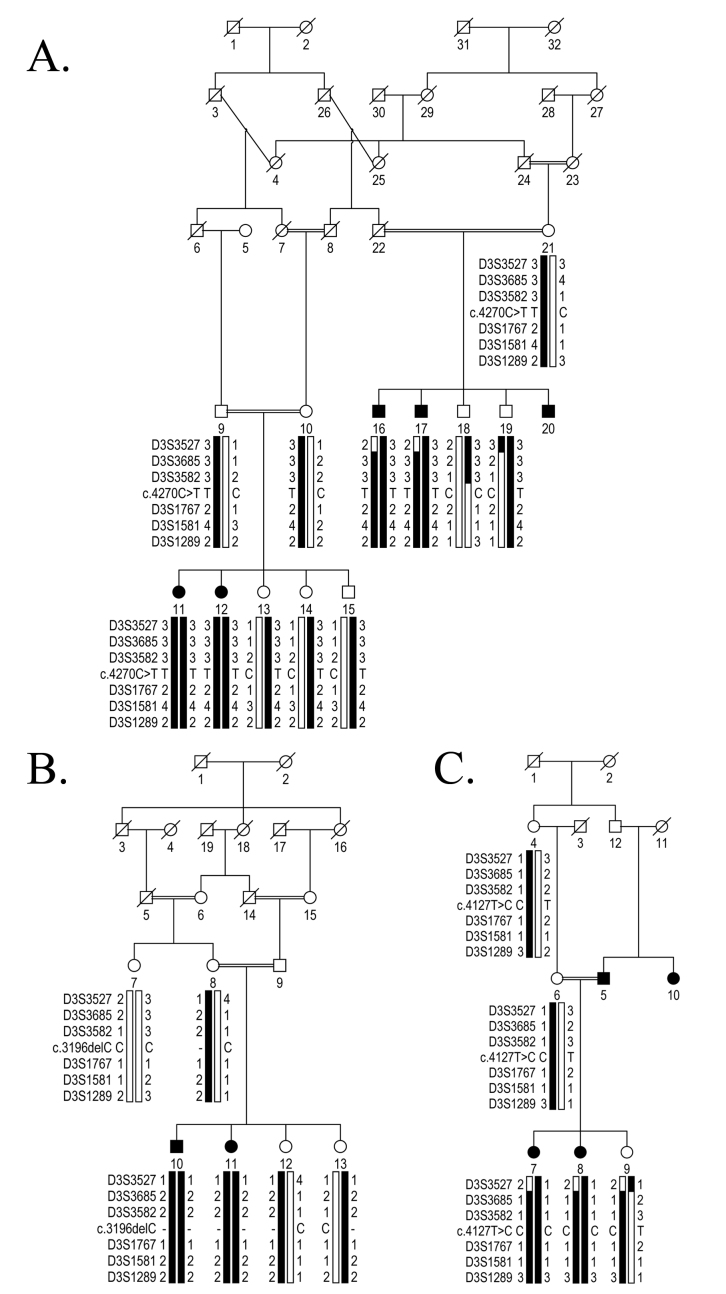
Figure 1.
Genetic analysis of chromosome 3p21-linked pedigrees harboring mutations in FYCO1. A: Pedigree illustrating the segregation of a single base substitution (c.4270C>T; p.Arg1424Ter) in all available affected and unaffected members of PKCC193. B: Pedigree illustrating the segregation of a single base deletion (c.3196delC; p.His1066IlefsTer10) in all available affected and unaffected members of PKCC202. C: Illustration of a pedigree showing the segregation of a single base change (c.4127T>C; p.Leu1376Pro) in all available affected and unaffected members of PKCC220. The haplotypes of six 3p21 microsatellite markers are shown. The alleles forming the risk haplotype are in black, and the alleles not cosegregating with cataract are shown in white. Note: Squares: males; circles: females; filled symbols: affected individuals; double line between individuals: consanguinity; diagonal line through a symbol: deceased family member.
Table 1. Clinical characteristics of families PKCC193, PKCC202, and PKCC220 harboring mutations in FYCO1.
| Family ID | Individual ID | Sex | Age at first symptoms * | Age at enrollment | Visual Acuity (OD/OS) | Clinical Findings |
|---|---|---|---|---|---|---|
| PKCC193 |
11 |
F |
2.5 months |
7 years |
PL/PL |
B/L cataracts, B/L nystagmus |
| |
12 |
F |
4 months |
1 year |
PL/PL |
B/L cataracts, squint |
| |
16 |
M |
11 months |
32 years |
CF/CF |
B/L cataracts |
| |
17 |
M |
1.5 years |
36 years |
CF/CF |
B/L cataracts, B/L nystagmus |
| PKCC202 |
10 |
M |
4 months |
10 months |
CF/CF |
B/L cataracts |
| |
11 |
F |
3 months |
6.5 years |
No PL/CF |
B/L cataracts |
| PKCC220 |
7 |
F |
5 months |
4 years |
CF/CF |
B/L cataracts |
| 8 | F | 3 months | 9 months | CF/CF | B/L cataracts |
Abbreviations: CF, counting fingers; PL, light perception; B/L, bilateral; OD, oculus dextrus; OS, oculus sinister. * The age at first symptoms of cataracts (cloudiness) in affected individuals is according to the family medical records and/or information provided by the family elders.
Linkage analysis localized the disease interval to chromosome 3p harboring FYCO1 (Figure 1). Interestingly, FYCO1, a gene previously implicated in non-syndromic arCC in multiple Pakistani families [17], resides in the linkage interval. A maximum two-point LOD score of 3.06 (θ=0) was obtained with marker D3S3685 in PKCC193 (Table 2). A maximum two-point LOD score of 1.68 (θ=0) was obtained with marker D3S3582 in PKCC202 (Table 2). A maximum two-point LOD score of 1.34 (θ=0) was obtained with markers D3S3582 and D3S1289 in PKCC220 (Table 2).
Table 2. Two-point LOD scores of chromosome 3p microsatellite markers with alleles of families PKCC193, PKCC202, and PKCC220.
| ID | Markers | cM | Mb | 0 | 0.01 | 0.03 | 0.05 | 0.07 | 0.09 | 0.1 | 0.2 | 0.3 | Zmax | θmax |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PKCC193 |
D3S3527 |
63.12 |
39.3 |
−1.22 |
−0.91 |
−0.15 |
0 |
0.19 |
0.29 |
0.33 |
0.21 |
0.04 |
0.33 |
0.1 |
| |
D3S3685 |
67.94 |
42.5 |
3.06 |
3.06 |
2.99 |
2.85 |
2.71 |
2.57 |
2.43 |
2.36 |
1.67 |
3.06 |
0 |
| |
D3S3582 |
69.19 |
45.4 |
2.75 |
2.7 |
2.58 |
2.46 |
2.34 |
2.21 |
2.15 |
1.54 |
0.91 |
2.75 |
0 |
| |
D3S1767 |
69.9 |
47 |
1.7 |
1.64 |
1.53 |
1.43 |
1.33 |
1.23 |
1.16 |
0.63 |
0.21 |
1.7 |
0 |
| |
D3S1581 |
70.61 |
48.6 |
2.78 |
2.73 |
2.5 |
2.38 |
2.26 |
2.05 |
1.99 |
1.22 |
0.55 |
2.78 |
0 |
| |
D3S1289 |
71.41 |
54.5 |
1.29 |
1.25 |
1.17 |
1.13 |
1.05 |
1.04 |
0.97 |
0.65 |
0.35 |
1.29 |
0 |
| PKCC202 |
D3S3527 |
63.12 |
39.3 |
0.69 |
0.68 |
0.66 |
0.63 |
0.61 |
0.57 |
0.56 |
0.38 |
0.2 |
0.69 |
0 |
| |
D3S3685 |
67.94 |
42.5 |
0.68 |
0.66 |
0.64 |
0.61 |
0.58 |
0.54 |
0.52 |
0.35 |
0.18 |
0.68 |
0 |
| |
D3S3582 |
69.19 |
45.4 |
1.68 |
1.63 |
1.54 |
1.44 |
1.34 |
1.24 |
1.19 |
0.72 |
0.33 |
1.68 |
0 |
| |
D3S1767 |
69.9 |
47 |
0.16 |
0.16 |
0.15 |
0.15 |
0.14 |
0.14 |
0.13 |
0.1 |
0.06 |
0.16 |
0 |
| |
D3S1581 |
70.61 |
48.6 |
1.63 |
1.59 |
1.49 |
1.39 |
1.29 |
1.2 |
1.15 |
0.69 |
0.31 |
1.63 |
0 |
| |
D3S1289 |
71.41 |
54.5 |
0.68 |
0.66 |
0.64 |
0.61 |
0.58 |
0.54 |
0.52 |
0.35 |
0.18 |
0.68 |
0 |
| PKCC220 |
D3S3527 |
63.12 |
39.3 |
-∞ |
−1.64 |
−1.14 |
−0.90 |
−0.74 |
−0.62 |
−0.57 |
−0.25 |
−0.10 |
−0.10 |
0.3 |
| |
D3S3685 |
67.94 |
42.5 |
1.03 |
1 |
0.94 |
0.89 |
0.83 |
0.77 |
0.74 |
0.47 |
0.22 |
1.03 |
0 |
| |
D3S3582 |
69.19 |
45.4 |
1.34 |
1.32 |
1.26 |
1.2 |
1.15 |
1.09 |
1.06 |
0.78 |
0.5 |
1.34 |
0 |
| |
D3S1767 |
69.9 |
47 |
1.03 |
1 |
0.94 |
0.89 |
0.83 |
0.77 |
0.74 |
0.47 |
0.22 |
1.03 |
0 |
| |
D3S1581 |
70.61 |
48.6 |
0.24 |
0.23 |
0.21 |
0.19 |
0.18 |
0.16 |
0.15 |
0.08 |
0.03 |
0.24 |
0 |
| D3S1289 | 71.41 | 54.5 | 1.34 | 1.32 | 1.26 | 1.2 | 1.15 | 1.09 | 1.06 | 0.78 | 0.5 | 1.34 | 0 |
Next, we sequenced all coding exons and the exon–intron junctions of FYCO1 in all three families. We identified a novel homozygous substitution (c.4270C>T) in PKCC193 (Figure 2A,B). This homozygous substitution results in premature termination of the FYCO1 protein by changing arginine at position 1424 into a stop codon (p.Arg1424Ter; Figure 2A,B). All affected individuals of PKCC193 are homozygous for this variation, whereas unaffected individuals are either heterozygous or homozygous for the wild-type allele (Figure 1A). This variant (c.4270C>T; p.Arg1424Ter) was identified in the heterozygous state in three different population databases with a global minor allele frequency (MAF) of 0.000008 (gnomAD), 0.000008 (ExAC), and 0.00002 (TOPMed) in two individuals of non-Finnish European descent, a single African individual, and three individuals of a study-wide group, respectively. We did not find the c.4270C>T mutation in the Asian population and in 96 ethnically matched control individuals.
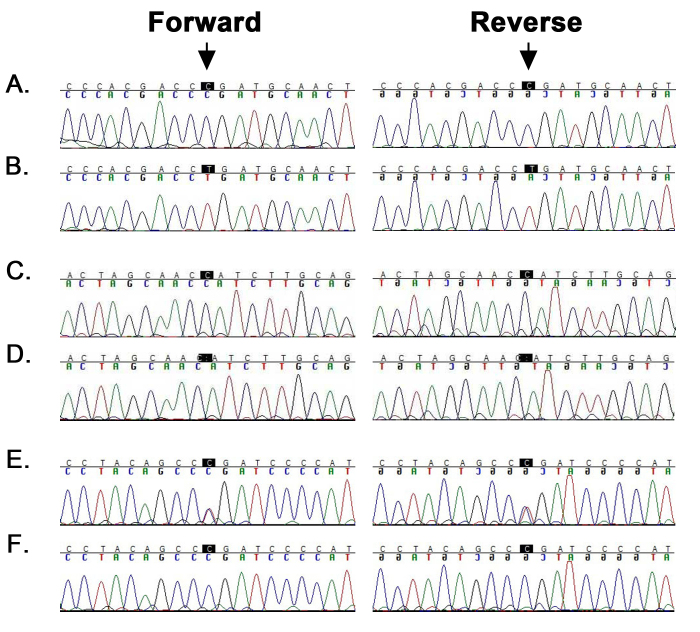
Figure 2.
Bidirectional Sanger sequencing identified mutations in FYCO1 in chromosome 3p21-linked pedigrees. A, B: Forward and reverse sequence chromatograms of individual 18 (unaffected) harboring the wild-type allele and individual 11 (affected) homozygous for a single base change: c.4270C>T (p.Arg1424Ter) in PKCC193. C, D: Forward and reverse sequence chromatograms of individual 7 (unaffected) harboring the wild-type allele and individual 10 (affected) homozygous for a single base deletion: c.3196delC (p.His1066IlefsTer10) in PKCC202. E, F: Forward and reverse sequence chromatograms of individual 6 (unaffected) heterozygous for a single base change and individual 7 (affected) homozygous for a single base substitution: c.4127T>C (p.Leu1376Pro) in PKCC220. Note: The arrows point to the base-pair substitution or deletion identified in each pedigree.
In PKCC202, we identified a novel homozygous single-base deletion (c.3196delC) in FYCO1 resulting in a frameshift mutation and premature truncation of the protein (p.His1066IlefsTer10; Figure 2C,D). The p.His1066IlefsTer10 variant showed segregation with the disease phenotype in all affected and unaffected individuals of PKCC202 (Figure 1B) and was not identified in the 1000 Genomes, ExAC browser, Exome Variant Server, and dbSNP databases. Moreover, the variant was also absent in 96 ethnically matched control individuals. In addition to novel variants, we identified a reported missense variant (c.4127T>C, p.Leu1376Pro) in PKCC220 (Figure 2E,F). The variant revealed complete segregation with the disease phenotype in all available affected and unaffected individuals of PKCC220 (Figure 1C) and was not present in 96 ethnically matched control individuals.
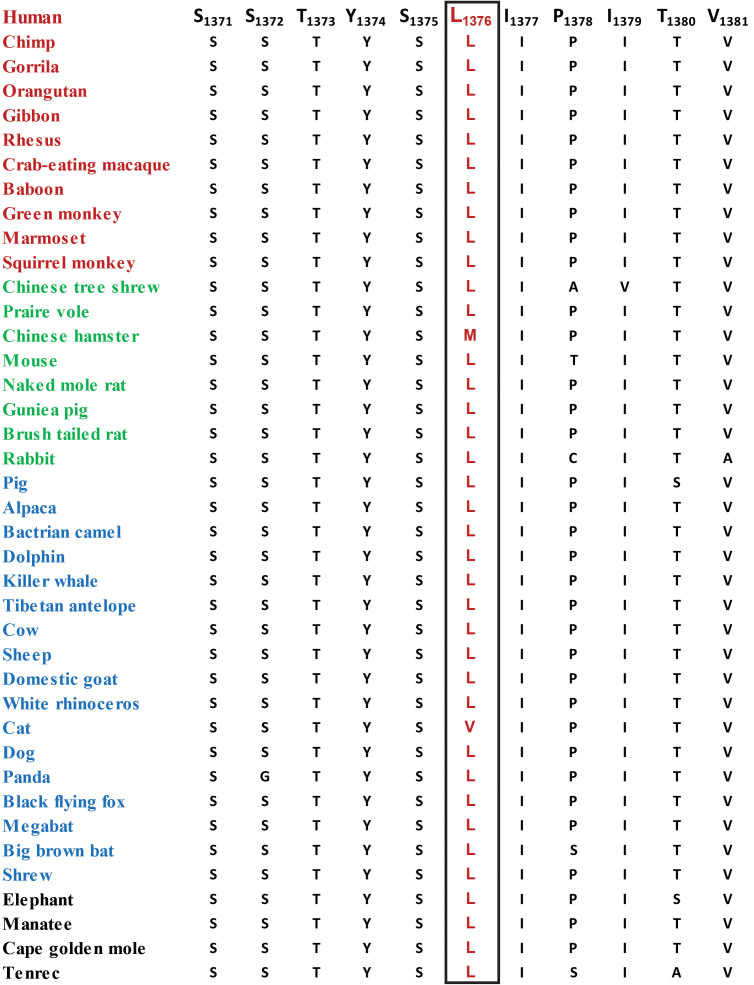
In contrast to the two novel mutations, i.e., the frameshift (c.3196delC; p.His1066IlefsTer10) and nonsense (c.4270C>T; p.Arg1424Ter) that are predicted to result in truncated FYCO1 proteins, the transcript harboring the previously reported missense (c.4127T>C, p.Leu1376Pro) allele is expected to produce a full-length FYCO1 protein. Importantly, amino acid leucine 1376 (in FYCO1) and the amino acids in the immediate neighborhood are well conserved in FYCO1 orthologs (Figure 3). We next examined the effect of leucine substitution on the FYCO1 protein with in silico analysis. PolyPhen-2, Mutation Assessor, MutationTaster, and SIFT algorithms were suggestive of probably damaging, low impact, disease-causing, and deleterious, respectively. Taken together, evolutionary conservation and in silico analysis suggest that the proline substitution would be detrimental to the native structure, and most likely, the physiological function of the FYCO1 protein.
Figure 3.
Sequence alignment of FYCO1 orthologs illustrating the conservation of amino acid leucine at position 1376. The boxed amino acids illustrate the conservation of Leu1376 among other FYCO1 orthologs. Red: primates; green: Euarchontoglires; blue: Laurasiatheria; black: Afrotheria.
Discussion
We report two novel and a previously reported mutation in FYCO1 associated with non-syndromic autosomal recessive cataracts in three unrelated consanguineous familial cases. The ophthalmic examination confirmed cataracts in all three families. The STR marker-based linkage analysis localized the critical interval to chromosome 3p with maximum two-point LOD scores of 3.06, 1.68, and 1.34 at θ=0 for PKCC193, PKCC202, and PKCC220, respectively (Table 2). Sequencing of the coding exons of FYCO1 identified two novel and a reported mutation that segregated with the disease phenotype in all three families and was absent in control individuals. Taken together, these results strongly suggest that mutations in FYCO1 are responsible for recessive congenital cataracts in PKCC193, PKCC202, and PKCC220.
FYCO1 is a member of the PI(3)P-binding protein family localized to autophagosomes and mediates transport of microtubule plus-end-directed vesicles [47]. The domain structure of FYCO1 comprises an α-helical RUN domain, four long coiled-coil regions, an FYVE zinc-finger domain, an LC3-interacting region (LIR), and a Golgi dynamics (GOLD) domain [17,48]. Pras et al. first reported a novel locus CATC2 (cataract, autosomal recessive congenital 2, OMIM: 610019) mapped in three consanguineous Arab families to the short arm of chromosome 3 [49]. Subsequently, Chen and colleagues mapped additional multiple familial cases with arCC at chromosome 3p overlapping with the CATC2 locus and identified mutations in FYCO1 in 12 Pakistani and one Arab family [17].
To date, a total of 19 mutations have been reported in FYCO1, including 11 mutations in the coiled-coil region of FYCO1 (Table 3). In another study, Chen and colleagues reported two homozygous variants (c.2345delA; p.Gln782ArgfsTer32 and c.3151–2A>C; p.Ala1051AspfsTer27) implicated in arCC in Pakistani families [18]. Recently, multiple studies reported mutations in FYCO1 implicated in arCC in Saudi (c.2506delG; p.Ala836ProfsTer80 and c.449T>C; p.Ile150Thr), Egyptian (c.2206C>T; p.Gln736Ter), and British (c.3670C>T; p.Arg1224Ter and c.3945–1G>C) familial and sporadic cases [34,50-52]. Moreover, two compound heterozygous variants in FYCO1 have been reported from Saudi Arabia and China [53,54]. Two homozygous mutations in FYCO1 have also been identified in Iranian and Russian familial cases [55,56].
Table 3. Summary of cataract-causing mutations identified in FYCO1.
| Exon/ Intron | DNA Change | Protein Change | Type | Population | Reference |
|---|---|---|---|---|---|
| Ex6 |
c.449T>C |
p.I150T |
Missense |
KSA |
34 |
| Ex8 |
c.808C>T and IVS12; c.3587+1G>T |
p.Q270X/ splice variant |
Compound heterozygous |
China |
53 |
| Ex8 |
c.1045C>T |
p.Q349X |
Nonsense |
Pakistan |
17 |
| Ex8 |
c.l056_1071delGGCCACACGGGACTCA |
p.E352DfsX9 |
Frameshift |
Iran |
56 |
| Ex8 |
c.1546C>T |
p.Q516X |
Nonsense |
Israel |
17 |
| Ex8 |
c.1621C>T |
p.Q541X |
Nonsense |
Russia |
55 |
| Ex8 |
c.2206C>T |
p.Q736X |
Nonsense |
Pakistan |
17 |
| Ex8 |
c.2206C>T |
p.Q736X |
Nonsense |
Egypt |
50 |
| Ex8 |
c.2206C>T |
p.Q736X |
Nonsense |
Pakistan |
18 |
| Ex8 |
c.2345delA |
p.Q782RfsX32 |
Frameshift |
Pakistan |
18 |
| Ex8 |
c.2345delA/ c.2714_2715delCA |
p.Q782RfsX32/ p.T905SfsX2 |
Compound heterozygous |
KSA/ UAE |
54 |
| Ex8 |
c.2506delG |
p.A836PfsX80 |
Frameshift |
KSA |
51 |
| Ex8 |
c.2761C>T |
p.R921X |
Nonsense |
Pakistan |
17 |
| Ex8 |
c.2830C>T |
p.R944X |
Nonsense |
Pakistan |
17 |
| IVS9 |
c.3150+1G>T |
Splice variant |
Splice variant |
Pakistan |
17 |
| IVS9 |
c.3151–2A>C |
p.A1051DfsX27 |
Frameshift |
Pakistan |
18 |
| Ex10 |
c.3196delC |
p.H1066IfsX10 |
Frameshift |
Pakistan |
This Study |
| Ex13 |
c.3670C>T |
p.R1224X |
Nonsense |
UK |
52 |
| Ex13 |
c.3755delC |
p.A1252DfsX71 |
Frameshift |
Pakistan |
17 |
| Ex14 |
c.3858_3862dupGGAAT |
p.L1288WfsX37 |
Frameshift |
Pakistan |
17 |
| IVS14 |
c.3945–1G>C |
Splice variant |
Splice variant |
UK |
52 |
| Ex16 |
c.4127T>C |
p.L1376P |
Missense |
Pakistan |
17 |
| Ex16 |
c.4127T>C |
p.L1376P |
Missense |
Pakistan |
This Study |
| Ex17 | c.4270C>T | p.R1424X | Nonsense | Pakistan | This Study |
Note: KSA: Kingdom of Saudi Arabia; UAE: United Arab Emirates; UK: United Kingdom.
In conclusion, identification of multiple mutations in FYCO1 in diverse populations and the higher frequency of frameshift, splice, and nonsense mutations strongly suggest the significant contribution of FYCO1 in congenital cataracts. Moreover, the identification of mutations responsible for arCC in the present study further highlights the significant genetic contribution in familial cases of Pakistani descent, in general, and this cohort of arCC in particular, nearly 15% (19/129). This investigation will help to devise better strategies for identifying individuals at risk through genetic diagnosis leading to better cataract prevention.
Acknowledgments
The authors are thankful to all enrolled members for their participation in this study. The work was supported, in part, by the Higher Education Commission, Islamabad Pakistan, and the National Eye Institute Grant R01EY022714 (SAR).
References
- 1.Graw J. Genetics of crystallins: Cataract and beyond. Exp Eye Res. 2009;88:173–89. doi: 10.1016/j.exer.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 2.Hejtmancik JF, Kaiser-Kupfer MI, Piatigorsky J. Molecular biology and inherited disorders of the eye lens. In: Scriver CR, Beaudet AL, Sly WS, et al. (eds), The metabolic and molecular basis of inherited diseases. 8 ed. McGraw-Hill, New York; 2001:6033–6062. [Google Scholar]
- 3.Vrensen G, Kappelhof J, Willekens B. Morphology of the aging human lens. II. Ultrastructure of clear lenses. Lens Eye Toxic Res. 1990;7:1–30. [PubMed] [Google Scholar]
- 4.Bettelheim FA, Siew EL. Effect of change in concentration upon lens turbidity as predicted by the random fluctuation theory. Biophys J. 1983;41:29–33. doi: 10.1016/S0006-3495(83)84402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wood AM, Truscott RJ. UV filters in human lenses: Tryptophan catabolism. Exp Eye Res. 1993;56:317–25. doi: 10.1006/exer.1993.1041. [DOI] [PubMed] [Google Scholar]
- 6.Benedek GB. Theory of transparency of the eye. Appl Opt. 1971;10:459–73. doi: 10.1364/AO.10.000459. [DOI] [PubMed] [Google Scholar]
- 7.al-Ghoul KJ, Costello MJ. Fiber cell morphology and cytoplasmic texture in cataractous and normal human lens nuclei. Curr Eye Res. 1996;15:533–42. doi: 10.3109/02713689609000764. [DOI] [PubMed] [Google Scholar]
- 8.Robinson GC, Jan JE, Kinnis C. Congenital ocular blindness in children, 1945 to 1984. 1502. Am J Dis Child. 1987;141:1321–4. doi: 10.1001/archpedi.1987.04460120087041. [DOI] [PubMed] [Google Scholar]
- 9.Foster A. Worldwide blindness, increasing but avoidable. Semin Ophthalmol. 1993;8:166–70. [Google Scholar]
- 10.Gilbert C, Foster A. Childhood blindness in the context of VISION 2020-the right to sight. Bull World Health Organ. 2001;79:227–32. [PMC free article] [PubMed] [Google Scholar]
- 11.Rahi JS, Sripathi S, Gilbert CE, Foster A. Childhood blindness in India: causes in 1318 blind school students in nine states. Eye (Lond) 1995;9:545–50. doi: 10.1038/eye.1995.137. [DOI] [PubMed] [Google Scholar]
- 12.Apple DJ, Ram J, Foster A, Peng Q. Elimination of cataract blindness: a global perspective entering the new millenium. Surv Ophthalmol. 2000;45(Suppl 1):S1–196. [PubMed] [Google Scholar]
- 13.Butt IA, Jalis M, Waseem S, Moqeet A, Inam-ul-Haq M. Spectrum of congenital and developmental anomalies of eye. Al Shifa J Ophthalmol. 2007;3:56–60. [Google Scholar]
- 14.Dineen B, Bourne RR, Jadoon Z, Shah SP, Khan MA, Foster A, Gilbert CE, Khan MD. Causes of blindness and visual impairment in Pakistan. The Pakistan national blindness and visual impairment survey. Br J Ophthalmol. 2007;91:1005–10. doi: 10.1136/bjo.2006.108035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aldahmesh MA, Khan AO, Mohamed JY, Alghamdi MH, Alkuraya FS. Identification of a truncation mutation of acylglycerol kinase (AGK) gene in a novel autosomal recessive cataract locus. Hum Mutat. 2012;33:960–2. doi: 10.1002/humu.22071. [DOI] [PubMed] [Google Scholar]
- 16.Ansar M. Chung Hl, Taylor RL, Nazir A, Imtiaz S, Sarwar MT, Manousopoulou A, Makrythanasis P, Saeed S, Falconnet E. Bi-allelic Loss-of-Function Variants in DNMBP Cause Infantile Cataracts. Am J Hum Genet. 2018;103:568–78. doi: 10.1016/j.ajhg.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Ma Z, Jiao X, Fariss R, Kantorow WL, Kantorow M, Pras E, Frydman M, Pras E, Riazuddin S, Riazuddin SA, Hejtmancik JF. Mutations in FYCO1 cause autosomal-recessive congenital cataracts. Am J Hum Genet. 2011;88:827–38. doi: 10.1016/j.ajhg.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Wang Q, Cabrera PE, Zhong Z, Sun W, Jiao X, Chen Y, Govindarajan G, Naeem MA, Khan SN, Ali MH, Assir MZ, Rahman FU, Qazi ZA, Riazuddin S, Akram J, Riazuddin SA, Hejtmancik JF. Molecular Genetic Analysis of Pakistani Families With Autosomal Recessive Congenital Cataracts by Homozygosity Screening. Invest Ophthalmol Vis Sci. 2017;58:2207–17. doi: 10.1167/iovs.17-21469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cohen D, Bar-Yosef U, Levy J, Gradstein L, Belfair N, Ofir R, Joshua S, Lifshitz T, Carmi R, Birk OS. Homozygous CRYBB1 deletion mutation underlies autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci. 2007;48:2208–13. doi: 10.1167/iovs.06-1019. [DOI] [PubMed] [Google Scholar]
- 20.Evers C, Paramasivam N, Hinderhofer K, Fischer C, Granzow M, Schmidt-Bacher A, Eils R, Steinbeisser H, Schlesner M, Moog U. SIPA1L3 identified by linkage analysis and whole-exome sequencing as a novel gene for autosomal recessive congenital cataract. Eur J Hum Genet. 2015;23:1627–33. doi: 10.1038/ejhg.2015.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiaox X, Khan SY, Irum B, Khan AO, Wang Q, Kabir F, Khan AA, Husnain T, Akram J, Riazuddin S, Hejtmancik JF, Riazuddin SA. Missense Mutations in CRYAB Are Liable for Recessive Congenital Cataracts. PLoS One. 2015;10:e0137973. doi: 10.1371/journal.pone.0137973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaul H, Riazuddin SA, Shahid M, Kousar S, Butt NH, Zafar AU, Khan SN, Husnain T, Akram J, Hejtmancik JF, Riazuddin S. Autosomal recessive congenital cataract linked to EPHA2 in a consanguineous Pakistani family. Mol Vis. 2010;16:511–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Khan SY, Vasanth S, Kabir F, Gottsch JD, Khan AO, Chaerkady R, Lee MC, Leitch CC, Ma Z, Laux J, Villasmil R, Khan SN, Riazuddin S, Akram J, Cole RN, Talbot CC, Pourmand N, Zaghloul NA, Hejtmancik JF, Riazuddin SA. FOXE3 contributes to Peters anomaly through transcriptional regulation of an autophagy-associated protein termed DNAJB1. Nat Commun. 2016;7:10953. doi: 10.1038/ncomms10953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science. 2011;331:1571–6. doi: 10.1126/science.1195970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ponnam SP, Ramesha K, Tejwani S, Ramamurthy B, Kannabiran C. Mutation of the gap junction protein alpha 8 (GJA8) gene causes autosomal recessive cataract. J Med Genet. 2007;44:e85. doi: 10.1136/jmg.2007.050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ponnam SP, Ramesha K, Tejwani S, Matalia J, Kannabiran C. A missense mutation in LIM2 causes autosomal recessive congenital cataract. Mol Vis. 2008;14:1204–8. [PMC free article] [PubMed] [Google Scholar]
- 27.Pras E, Frydman M, Levy-Nissenbaum E, Bakhan T, Raz J, Assia EI, Goldman B, Pras E. A nonsense mutation (W9X) in CRYAA causes autosomal recessive cataract in an inbred Jewish Persian family. Invest Ophthalmol Vis Sci. 2000;41:3511–5. [PubMed] [Google Scholar]
- 28.Pras E, Raz J, Yahalom V, Frydman M, Garzozi HJ, Pras E, Hejtmancik JF. A nonsense mutation in the glucosaminyl (N-acetyl) transferase 2 gene (GCNT2): association with autosomal recessive congenital cataracts. Invest Ophthalmol Vis Sci. 2004;45:1940–5. doi: 10.1167/iovs.03-1117. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran RD, Perumalsamy V, Hejtmancik JF. Autosomal recessive juvenile onset cataract associated with mutation in BFSP1. Hum Genet. 2007;121:475–82. doi: 10.1007/s00439-006-0319-6. [DOI] [PubMed] [Google Scholar]
- 30.Riazuddin SA, Yasmeen A, Yao W, Sergeev YV, Zhang Q, Zulfiqar F, Riaz A, Riazuddin S, Hejtmancik JF. Mutations in betaB3-crystallin associated with autosomal recessive cataract in two Pakistani families. Invest Ophthalmol Vis Sci. 2005;46:2100–6. doi: 10.1167/iovs.04-1481. [DOI] [PubMed] [Google Scholar]
- 31.Smaoui N, Beltaief O, BenHamed S, M’Rad R, Maazoul F, Ouertani A, Chaabouni H, Hejtmancik JF. A homozygous splice mutation in the HSF4 gene is associated with an autosomal recessive congenital cataract. Invest Ophthalmol Vis Sci. 2004;45:2716–21. doi: 10.1167/iovs.03-1370. [DOI] [PubMed] [Google Scholar]
- 32.Yasmeen A, Riazuddin SA, Kaul H, Mohsin S, Khan M, Qazi ZA, Nasir IA, Zafar AU, Khan SN, Husnain T, Akram J, Hejtmancik JF, Riazuddin S. Autosomal recessive congenital cataract in consanguineous Pakistani families is associated with mutations in GALK1. Mol Vis. 2010;16:682–8. [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao L, Chen XJ, Zhu J, Xi YB, Yang X, Hu LD, Ouyang H, Patel SH, Jin X, Lin D, Wu F, Flagg K, Cai H, Li G, Cao G, Lin Y, Chen D, Wen C, Chung C, Wang Y, Qiu A, Yeh E, Wang W, Hu X, Grob S, Abagyan R, Su Z, Tjondro HC, Zhao XJ, Luo H, Hou R, Jefferson J, Perry P, Gao W, Kozak I, Granet D, Li Y, Sun X, Wang J, Zhang L, Liu Y, Yan YB, Zhang K. Lanosterol reverses protein aggregation in cataracts. Nature. 2015;523:607–11. doi: 10.1038/nature14650. [DOI] [PubMed] [Google Scholar]
- 34.Khan AO, Aldahmesh MA, Alkuraya FS. Phenotypes of recessive pediatric cataract in a cohort of children with identified homozygous gene mutations (An American Ophthalmological Society Thesis). Transactions of the American Ophthalmological Society. 2015;113. [PMC free article] [PubMed] [Google Scholar]
- 35.Micheal S. Niewold ITsGl, Siddiqui SN, Zafar SN, Khan MI, Bergen AA. Delineation of Novel Autosomal Recessive Mutation in GJA3 and Autosomal Dominant Mutations in GJA8 in Pakistani Congenital Cataract Families. Genes (Basel) 2018;9:112. doi: 10.3390/genes9020112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Forshew T, Johnson CA, Khaliq S, Pasha S, Willis C, Abbasi R, Tee L, Smith U, Trembath RC, Mehdi SQ, Moore AT, Maher ER. Locus heterogeneity in autosomal recessive congenital cataracts: linkage to 9q and germline HSF4 mutations. Hum Genet. 2005;117:452–9. doi: 10.1007/s00439-005-1309-9. [DOI] [PubMed] [Google Scholar]
- 37.Kaul H, Riazuddin SA, Yasmeen A, Mohsin S, Khan M, Nasir IA, Khan SN, Husnain T, Akram J, Hejtmancik JF, Riazuddin S. A new locus for autosomal recessive congenital cataract identified in a Pakistani family. Mol Vis. 2010;16:240–5. [PMC free article] [PubMed] [Google Scholar]
- 38.Riazuddin SA, Yasmeen A, Zhang Q, Yao W, Sabar MF, Ahmed Z, Riazuddin S, Hejtmancik JF. A new locus for autosomal recessive nuclear cataract mapped to chromosome 19q13 in a Pakistani family. Invest Ophthalmol Vis Sci. 2005;46:623–6. doi: 10.1167/iovs.04-0955. [DOI] [PubMed] [Google Scholar]
- 39.Sabir N, Riazuddin SA, Kaul H, Iqbal F, Nasir IA, Zafar AU, Qazi ZA, Butt NH, Khan SN, Husnain T, Hejtmancik JF, Riazuddin S. Mapping of a novel locus associated with autosomal recessive congenital cataract to chromosome 8p. Mol Vis. 2010;16:2911–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Sabir N, Riazuddin SA, Butt T, Iqbal F, Nasir IA, Zafar AU, Qazi ZA, Butt NH, Khan SN, Husnain T, Hejtmancik JF, Riazuddin S. Mapping of a new locus associated with autosomal recessive congenital cataract to chromosome 3q. Mol Vis. 2010;16:2634–8. [PMC free article] [PubMed] [Google Scholar]
- 41.Bassnett S, Shi Y, Vrensen GF. Biological glass: structural determinants of eye lens transparency. Philos Trans R Soc Lond B Biol Sci. 2011;366:1250–64. doi: 10.1098/rstb.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bloemendal H, de Jong W, Jaenicke R, Lubsen NH, Slingsby C, Tardieu A. Ageing and vision: Structure, stability and function of lens crystallins. Prog Biophys Mol Biol. 2004;86:407–85. doi: 10.1016/j.pbiomolbio.2003.11.012. [DOI] [PubMed] [Google Scholar]
- 43.Moreau KL, King JA. Protein misfolding and aggregation in cataract disease and prospects for prevention. Trends Mol Med. 2012;18:273–82. doi: 10.1016/j.molmed.2012.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hejtmancik JF. Congenital cataracts and their molecular genetics. Semin Cell Dev Biol. 2008;19:134–49. doi: 10.1016/j.semcdb.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lathrop GM, Lalouel JM. Easy calculations of lod scores and genetic risks on small computers. Am J Hum Genet. 1984;36:460–5. [PMC free article] [PubMed] [Google Scholar]
- 46.Schaffer AA, Gupta SK, Shriram K, Cottingham RW. Avoiding recomputation in genetic linkage analysis. Hum Hered. 1994;44:225–37. doi: 10.1159/000154222. [DOI] [PubMed] [Google Scholar]
- 47.Rose A, Schraegle SJ, Stahlberg EA, Meier I. Coiled-coil protein composition of 22 proteomes-differences and common themes in subcellular infrastructure and traffic control. BMC Evol Biol. 2005;5:66. doi: 10.1186/1471-2148-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ma J, Becker C, Reyes C, Underhill DM. Cutting edge: FYCO1 recruitment to dectin-1 phagosomes is accelerated by light chain 3 protein and regulates phagosome maturation and reactive oxygen production. J Immunol. 2014;192:1356–60. doi: 10.4049/jimmunol.1302835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pras E, Pras E, Bakhan T, Levy-Nissenbaum E, Lahat H, Assia EI, Garzozi HJ, Kastner DL, Goldman B, Frydman M. A gene causing autosomal recessive cataract maps to the short arm of chromosome 3. Isr Med Assoc J. 2001;3:559–62. [PubMed] [Google Scholar]
- 50.Abouzeid H, Helmy G, Sada ME, Sherif M, Yacoub MH, Boisset G, Favez T, Schorderet DF. FYCO1 mutation hotspot in congenital cataract. Invest Ophthalmol Vis Sci. 2012;53:1723. [Google Scholar]
- 51.Aldahmesh MA, Khan AO, Mohamed JY, Hijazi H, Al-Owain M, Alswaid A, Alkuraya FS. Genomic analysis of pediatric cataract in Saudi Arabia reveals novel candidate disease genes. Genet Med. 2012;14:955–62. doi: 10.1038/gim.2012.86. [DOI] [PubMed] [Google Scholar]
- 52.Gillespie RL, O’Sullivan J, Ashworth J, Bhaskar S, Williams S, Biswas S, Kehdi E, Ramsden SC, Clayton-Smith J, Black GC, Lloyd IC. Personalized diagnosis and management of congenital cataract by next-generation sequencing. Ophthalmology. 2014;121:2124–37. doi: 10.1016/j.ophtha.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 53.Li J, Leng Y, Han S, Yan L, Lu C, Luo Y, Zhang X, Cao L. Clinical and genetic characteristics of Chinese patients with familial or sporadic pediatric cataract. Orphanet J Rare Dis. 2018;13:94. doi: 10.1186/s13023-018-0828-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel N, Anand D, Monies D, Maddirevula S, Khan AO, Algoufi T, Alowain M, Faqeih E, Alshammari M, Qudair A, Alsharif H, Aljubran F, Alsaif HS, Ibrahim N, Abdulwahab FM, Hashem M, Alsedairy H, Aldahmesh MA, Lachke SA, Alkuraya FS. Novel phenotypes and loci identified through clinical genomics approaches to pediatric cataract. Hum Genet. 2017;136:205–25. doi: 10.1007/s00439-016-1747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Barashkov NA, Konovalov FA, Teryutin FM, Solovyev AV, Pshennikova VG, Romanov GP, Gotovtsev NN, Morozov IV, Bondar AA, Dzhemileva LU, Khusnutdinova EK, Alexeev AN, Tomsky MI, Posukh OL, Fedorova SA. Whole exome sequencing of the Yakut family from Eastern Siberia with congenital autosomal-recessive cataract: The novel nonsense mutation in the FYCO1 gene. Eur J Hum Genet. 2016;24:417. [Google Scholar]
- 56.Özkan EG. Genetics of inherited eye diseases in the Iranian population. Doctoral thesis. St George's, University of London. 2016. [Google Scholar]