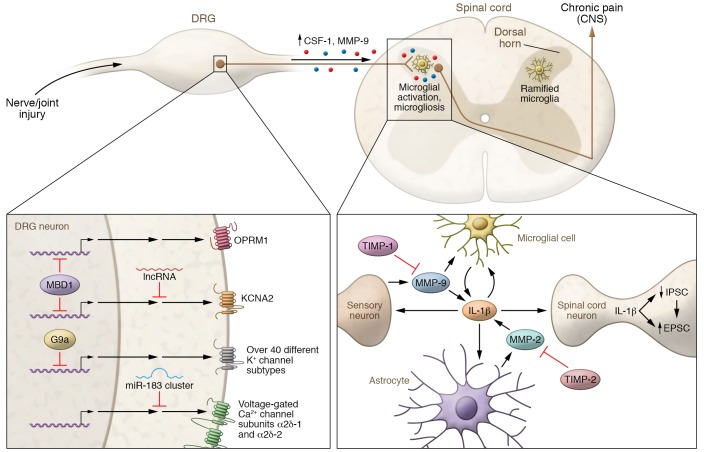
Figure 1. Pathogenesis of neuropathic pain and arthritic pain via gene regulation in DRG neurons and neuroinflammation in the spinal cord.
Lower left: Epigenetic regulation in DRG neurons in peripheral sensitization after nerve injury. In primary sensory neurons, MBD1 epigenetically suppresses expression of μ-opiod receptor and potassium channel subtype Kv1.2 (encoded by Kcna2). Kcna2 expression is also silenced by long noncoding RNA (lncRNA). Activity of G9a in DRG neurons increases following nerve injury, resulting in epigenetic silencing of more than 40 potassium channel subtypes. Voltage-gated calcium channel subunits α2δ-1 and α2δ-2, the molecular targets of gabapentin, are regulated by the miR-183 cluster. Right: Spinal cord microglia activation in chronic pain. Nerve injury and joint injury induce upregulation of MMP-9 and CSF-1 in DRG neurons. MMP-9 and CSF-1 undergo axonal transport to the spinal cord dorsal horn. Upon release, MMP-9 and CSF-1 induce microglia activation (e.g., p38 phosphorylation) and microgliosis (proliferation and morphological changes) in the ipsilateral spinal cord, leading to the development of chronic pain. Lower right: Spinal cord neuroinflammation in central sensitization and chronic pain. Upon activation, microglia produce and release IL-1β, which induces central sensitization and chronic pain via both presynaptic and postsynaptic regulations, leading to increased EPSCs and decreased IPSCs. IL-1β also modulates the activation of microglia and astrocytes in the spinal cord. Delayed but persistent MMP-2 production in astrocytes contributes to late-phase neuropathic pain. Both MMP-9 and MMP-2 are involved in regulating the cleavage and activation of IL-1β. Inhibition of MMP-9 and MMP-2 by TIMP-1 and TIMP-2 blocks neuropathic pain.