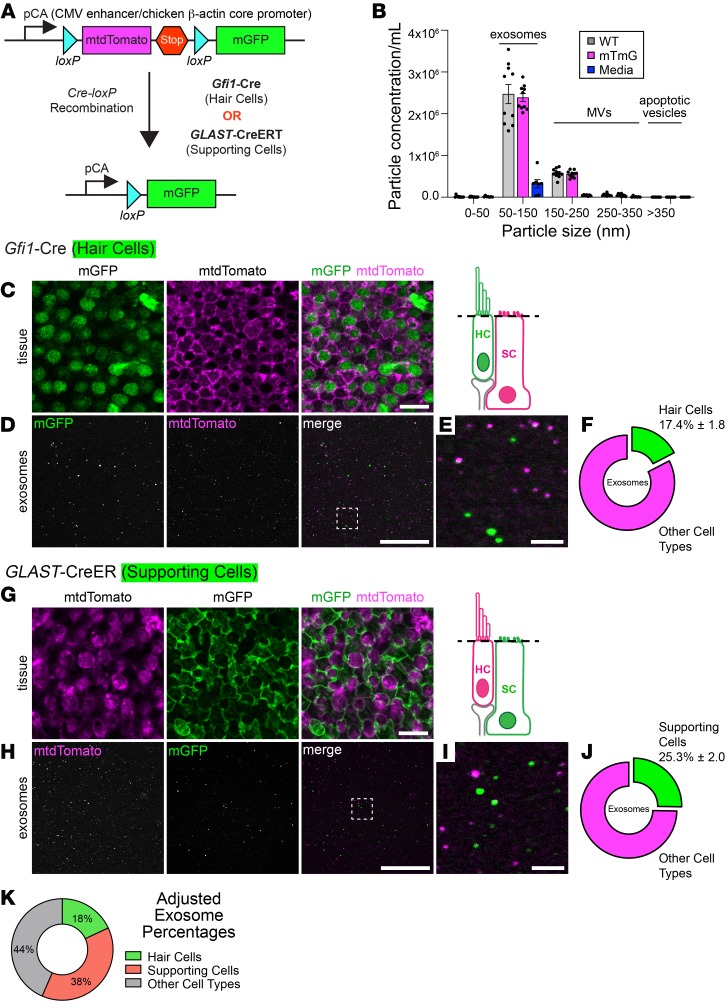
Figure 6. Supporting cells release more exosomes than hair cells under heat stress.
(A) mTmG double-fluorescent reporter mice constitutively express myristoylated tdTomato. When crossed with Cre recombinase–expressing mice, the loxP-flanked mtdTomato cassette is deleted in all Cre-expressing cells, resulting in tissue-specific mGFP fluorescence. (B) NTA of conditioned media from heat-shocked utricles of mTmG mice (magenta) or age-matched WT mice (gray) shows that lipidation of fluorophores in mTmG mice did not affect exosome release. The culture media (blue) contributed 10% of particles to each size category. MVs, microvesicles. Data indicate the mean ± SEM and are from 2 independent experiments (n = 5 NTA captures from 22 utricles for each condition). (C) Utricles from mTmG mice crossed with Gfi1-Cre mice displayed mGFP-expressing hair cells (green), whereas supporting cells retained mtdTomato expression (magenta). Schematic depicts the focal plane. (D) Fluorescence emitted from utricle-derived exosomes from mTmG mice crossed with Gfi1-Cre mice. Box indicates the region magnified in E. (F) 17.4% of utricle-derived exosomes in D were mGFP positive. (G) Supporting cells in mTmG mice crossed with GLAST-CreER mice were mGFP positive (green). All other cells retained mtdTomato expression (magenta). (H) Fluorescence emitted from utricle-derived exosomes from mTmG mice crossed with GLAST-CreER mice. Box indicates the region magnified in I. (J) 25.3% of exosomes visualized in H were mGFP positive. (K) Contributions of hair cells and supporting cells to the total utricle-derived exosome population after taking into account Cre recombinase efficiency in hair cells (Gfi1-Cre = 96.5%) and supporting cells (GLAST-CreER = 64.5%). Results showed that 44% of exosomes were probably contributed by other cell types. Data in F and J are presented as the mean ± SEM and are from 3 experiments (n = 9–11 utricles per condition). Scale bars: 10 μm (C and G), 50 μm (D and H), and 5 μm (E and I).