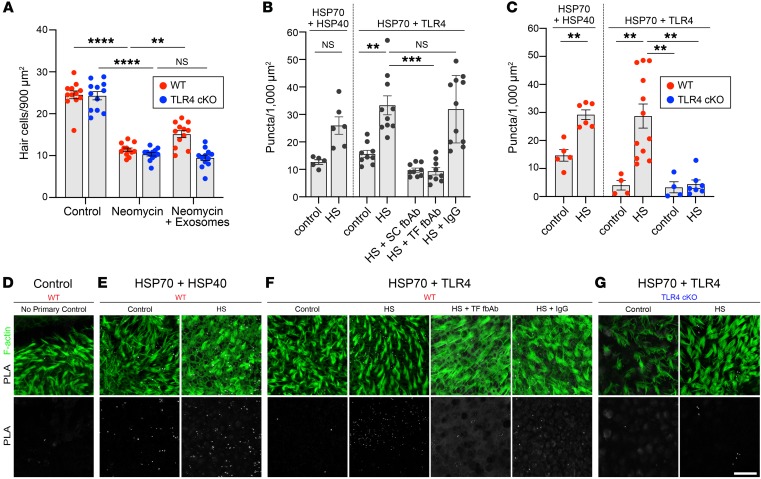
Figure 8. The protective effect of exosomes requires interaction of exosomal HSP70 with TLR4 on hair cells.
(A) Exosomes improved hair cell survival in neomycin-exposed utricles from control (WT) littermates (red) but not from hair cell–specific TLR4-cKO mice (blue). Data indicate the mean ± SEM (n = 12 utricles per condition). **P < 0.01 and ****P < 0.0001, by 2-way ANOVA with Holm-Šídák multiple comparisons test. (B and C) A PLA was performed to detect interaction between exosomal HSP70 and HSP40 or HSP70 and TLR4. (B) Two different fbAbs against HSP70 (HS) abolished the interaction between HSP70 and TLR4, whereas IgG had no effect. SC, Santa Cruz Biotechnology; TF, Thermo Fisher Scientific. (C) Heat shock increased the interaction between HSP70 and HSP40 and between HSP70 and TLR4 in WT utricles. Hair cell–specific deletion of TLR4 abolished the PLA signal in heat-shocked utricles from TLR4-cKO mice. Data in B and C indicate the mean ± SEM and are shown as the average number of puncta per 1000 μm2 (n = 4–12 utricles per condition). **P < 0.01 and ***P < 0.001, by Brown-Forsythe and Welsh ANOVA followed by Dunnett’s T3 multiple comparisons test. (D–G) Confocal images of PLA signals in utricles from WT (D–F) or TLR4-cKO (G) mice under control or heat shock conditions. Top row, F-actin (green) and PLA signal (white); bottom row, PLA signal only (white). (D) Negative control (no primary Ab). (E) Heat shock induced HSP70 interaction with HSP40 in WT utricles. (F) Heat shock increased HSP70 interaction with TLR4 in WT utricles. HSP70 fbAbs inhibited interaction between HSP70 and TLR4 in WT utricles, independently of heat shock, whereas control IgG had no effect. (G) Hair cell–specific deletion of TLR4 abolished the HSP70-TLR4 interaction in TLR4-cKO mice. Scale bar: 20 μm.