Abstract
ADP Ribosylation Factor 6 (ARF6) is a part of the RAS superfamily and regulates vesicular trafficking, remodeling of membrane lipids, and signaling pathways. Our previous study has found that ARF6, functioned as a downstream of Kras/ERK signaling pathway, could promote proliferation and Warburg effect in pancreatic cancer cells. Moreover, ARF6 is promising to be a biomarker for predicting prognosis of pancreatic cancer. Ferroptosis is a new defined iron-dependent form of nonapoptotic cell death, which is closely related to Kras mutation. Therefore, it is urgent to further explore the relationship between ARF6 and ferroptosis. Our study demonstrated that ARF6 did not directly regulate lipid peroxidation, but endowed pancreatic cancer cells to a status that is sensitive to oxidative stress, especially RSL3-induced lipid peroxidation. Further study revealed that ARF6 could also regulate gemcitabine resistance via multiple pathways. In conclusion, ARF6 has a profound effect on pancreatic cancer development.
Keywords: Ferroptosis, ARF6, ACSL4, gemcitabine, pancreatic cancer
Introduction
Ferroptosis is an iron-dependent form of nonapoptotic cell death, which is characterized by lipid peroxidation. Ferroptosis has been proved to link to diversified diseases, such as ischemia/reperfusion (I/R)-induced damage in organs, neuron degenerative diseases and so on [1,2]. However, ferroptosis was first detected in tumors. Brent R. Stockwell screened small molecules that could kill RAS mutant cancer cells and they found that some small molecules could activate an iron-dependent, nonapoptotic cell death in RAS mutant cancer cells, such as erastin and RAS-selective lethal 3 (RSL3) [3,4]. Ferroptosis is associated with a variety of physiological processes in tumor cells [5]. It is worth noting that ferroptosis is promising to be therapy target in cancers [6]. Recent researches also reported that ferroptosis could regulate cisplatin resistance or synergizes with cisplatin to effectively kill cancer cells [7-9].
Pancreatic cancer is a highly lethal tumor with an overall five-year survival rate of 6% (ranges from 2% to 9%) [10]. Gemcitabine is the base chemotherapy drug for pancreatic cancer [11]. Coincidentally, Kras gene is the most frequently mutated gene in pancreatic cancer, presenting in approximately 95% of pancreatic tumors [12]. Therefore, there might be some special links between ferroptosis and pancreatic cancer. However, the effect of ferroptosis on pancreatic cancer has not been fully investigated. Moreover, little literatures explored the relationship between ferroptosis and gemcitabine. Therefore, it is urgent to investigate the underlying effect of ferroptosis on pancreatic cancer and the promising value on cancer therapy.
ARF6 belongs to the Ras superfamily and encodes small guanine nucleotide-binding proteins (GTP-binding protein). ARF6 has comprehensive functions in cells, including assisting autophagy, activating lipid modifying enzymes, stimulating actin polymerization, innate immunity and so on [13]. Moreover, ARF6 regulated the invasion, metastasis and proliferation of cancer cells [14]. Particularly worth mentioning was that ARF6 directed downstream transport and recycled of cargo after clathrin-mediated endocytosis (CME) and clathrin-independent endocytosis (CIE) [15,16]. These indicated that ARF6 might have many potential functions that affected cell physiology by affecting the degradations and recycle of various functional proteins. Our previous study had revealed that Kras/ERK pathway could induce ARF6 and then a positive feedback loop was formed between ARF6 and c-myc, leading to the promotion of proliferation and Warburg effect in pancreatic cancer [17]. Recent study reported that c-myc could inhibit ferroptosis by regulating lipid metabolic gene expression [18]. Taken together, Kras and c-myc both regulate ferroptosis, then it is urgent to illustrate whether ARF6, which connects Kras to c-myc, regulates ferroptosis and this regulation exerts what significance for the development of pancreatic cancer.
In this study, we investigated the potential impact of ARF6 on ferroptosis in pancreatic cancer cells. We found that ARF6 did not directly regulate the generation of lipid peroxidation, but affected the sensitivity of RSL3-induced ferroptosis. As for mechanism, ARF6 decreased ACSL4 protein level and this effect endowed pancreatic cancer cells to a status that sensitized to oxidative stress. Further results revealed that ARF6 aggravated gemcitabine resistance by inhibiting ferroptosis and gemcitabine related metabolic proteins, DCK and hENT1.
Materials and methods
Cell culture
The human pancreatic duct epithelial cell line H6C7 and human pancreatic cancer cell lines AsPC-1, PANC-1, BxPC3, SW1990, Capan-1 and MIA PaCa-2 were obtained from ATCC and cultured according to standard ATCC protocols. In brief, H6C7, Capan-1 and PANC-1 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% fetal bovine serum (FBS). BxPC3 and AsPC-1 cells were cultured in Roswell Park Memorial Institute (PRMI) containing 10% FBS. SW1990 cells were cultured in Leibovitz (L-15) containing 10% FBS. MIA PaCa-2 cells were cultured in DMEM medium, with 10% FBS, 2.5% horse serum and 1% sodium pyruvate.
RNA isolation and quantitative real-time PCR
Total RNA was extracted using TRIzol reagent (Invitrogen, USA). Quantitative real-time PCR was conducted as described previously [19]. All the reactions were run in triplicate.
Primers used: Human ARF6: 5’-ATTACTACACCGGGACCCAGGGTCT-3’ (forward), 5’-AGGTCCTGCTTGTTGGCGAAGATG-3’ (reverse); Human ACSL4: 5’-CTGTTCAGCGTTTTGCAAGGTA-3’ (forward), 5’-TAGTGGCATCTCCCTGGTCC-3’ (reverse); Human hENT1: 5’-CCGGATGCTCACTCCAAAGT-3’ (forward), 5’-AATAACAGCAGGGGCAGCAT3’ (reverse); Human DCK: 5’-TCCATCGAAGGGAACATCGC-3’ (forward), 5’-CAGTGTCCTATGCAGGAGCC-3’ (reverse); Human β-actin: 5’-CTACGTCGCCCTGGACTTCGAGC-3’ (forward), 5’-GATGGAGCCGCCGATCCACACGG-3’ (reverse).
Western blot analysis
Western blot was performed as described previously [19]. ACSL4, GPx4 and SCD1 antibodies were purchased from Abcam. β-actin, hENT1, FSP1, DCK and RRM1 antibodies were purchased from Proteintech.
Lentivirus production
The pLKO.1 TRC cloning vector (Addgene plamid: 10878) was used to generate shRNA expression constructs against ARF6 as described previously [17]. Targets (21 bp) against ARF6 were 5’-GCTCACATGGTTAACCTCTAA-3’ and 5’-CAACAATCCTGTACAAGTTGA-3’. pLKO.1-shARF6 constructs, psPAX2 and pMD2.G were co-transfected into HEK-293T cells to generate lentiviral particles.
Small compounds
Erastin (HY-15763), RSL3 (HY-100218A), ROSI (HY-17386), OA (HY-N1446) and gemcitabine (HY-17026) were bought from MedChemExpress.
Cell viability
CCK-8 (Cell Counting Kit-8; Dojindo Laboratories) was used to detect cell viability according to the manufacturer’s instructions.
Lipid peroxidation assay
Lipid peroxidation was detected by BODIPY™ 581/591 C11 (D3861; ThermoFisher). In brief, cells, treated with test compounds for the indicated times, were harvested by trypsinization and then resuspended in 400 µL serum-free medium that contained BODIPY™ 581/591 C11 (2 µM). Next incubation for 30 minutes at 37°C in a cell culture incubator. Flow cytometer was then used to analyze these samples and data were collected from the FL1 channel. Per condition analyzed a minimum of 10,000 cells. Moreover, BODIPY™ 581/591 C11 (2 µM) also was directly incubated in cells that was adherent cultured for 30 minutes at 37°C, and then confocal microscopy was used to detect the fluorescence.
MDA assay
MDA was detected by Lipid Peroxidation MDA Assay Kit (S0131M; Beyoyime) according to the manufacturer’s instructions.
Immunohistochemical staining (IHC)
The clinical tissue samples were obtained from patients diagnosed with pancreatic cancer at Fudan University Shanghai Cancer Center, with patient consent and approval from the Institutional Research Ethics Committee. Antibodies against ARF6 and ACSL4 were used to conduct immunohistochemical staining in paraffin-embedded tissues according to standard IHC procedures. Anti-ACSL4 antibody (ab155282; Abcam) and Anti-ARF6 antibody (ab77581; Abcam) were used in a dilution factor of 1:100. Positive proportion and intensity were semiquantitatively scored as previously described [20].
Statistical analyses
All statistical analyses were performed using GraphPad Prism 8. Differences were considered significant at *, P < 0.05; **, P < 0.01.
Results
Knockdown of ARF6 enhances RSL3-induced ferroptosis
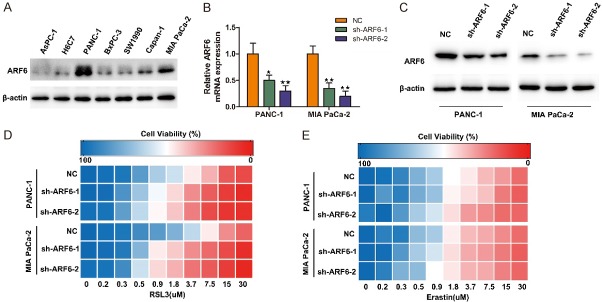
To select suitable cell lines for further investigation, we tested ARF6 protein level in human pancreatic duct epithelial cell line (H6C7) and several pancreatic cancer cell line. Our results demonstrated that ARF6 expression was relatively high in PANC-1 and MIA PaCa-2 cell lines (Figure 1A). Therefore, we next generated stable shRNA expression in PANC-1 and MIA PaCa-2 cell lines. The knockdown efficiency was further validated by quantitative RT-PCR and western blotting (Figure 1B and 1C). To determine whether ARF6 could regulate ferroptosis in pancreatic cancer cells, we tested cells’ sensitivity to two canonical ferroptosis inducers, RSL3 and erastin. Indeed, knockdown of ARF6 could robustly sensitize PANC-1 and MIA PaCa-2 cell lines to RSL3-induced ferroptosis, but not erastin-induced ferroptosis (Figure 1D and 1E). These results indicated that ARF6 could regulated RSL3-induced ferroptosis in pancreatic cancer cells.
Figure 1.
Knockdown of ARF6 enhances RSL3-induced ferroptosis. A. Western blotting analyzed ARF6 expression in multiple cell lines. B. qRT-PCR analyzed the knockdown efficiency of shARF6. C. Western blotting analyzed the knockdown efficiency of shARF6. D. Heat maps depicting the dose-dependent toxicity of RSL3 in PANC-1 and MIA PaCa-2 cell lines that transfected with shARF6 or shRNA-NC. E. Heat maps depicting the dose-dependent toxicity of erastin in PANC-1 and MIA PaCa-2 cell lines that transfected with shARF6 or shRNA-NC.
ARF6 does not cause lipid peroxidation but enhances RSL3-induced lipid peroxidation
One of the most important features of ferroptosis is lipid peroxidation, we then tested the level of malondialdehyde (MDA), a product of lipid peroxidation. Intriguingly, knockdown of ARF6 did not affect MDA level in both PANC-1 and MIA PaCa-2 cell lines but could enhance RSL3-induced MDA (Figure 2A and 2B). Next, we used a fluorescent probe, BODIPY™ 581/591 C11, to directly detect oxidized lipids. In this case, the fluorescence changes from red to green when this probe binds to oxidized lipids. We used flow cytometry to analyze the fluorescence and the results demonstrated that knockdown of ARF6 did not affect oxidized lipids level but promoted RSL3-induced oxidized lipids (Figure 2C and 2D). The results were further verified by confocal scanning imaging, RSL3 caused more oxidized lipids in ARF6 knockdown cells (Figure 2E).
Figure 2.
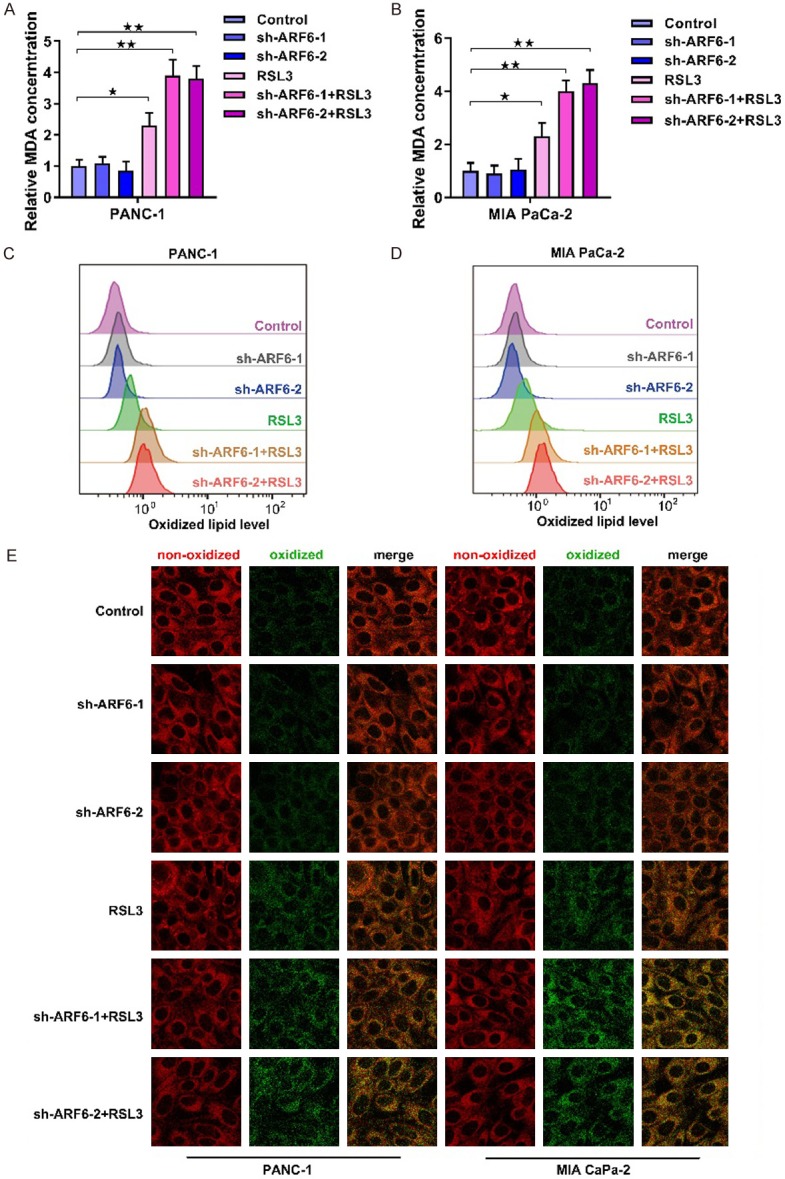
ARF6 does not cause lipid peroxidation but enhances RSL3-induced lipid peroxidation. A. MDA level was detected in AR6 silenced PANC-1 cells that pretreated RSL3 (2 uM) or not. B. MDA level was detected in AR6 silenced MIA PaCa-2 cells that pretreated with RSL3 or not. C. Flow cytometry analyzed the oxidized lipid in ARF6 silenced PANC-1 cells that pretreated with RSL3 (2 uM) or not. D. Flow cytometry analyzed the oxidized lipid in ARF6 silenced MIA PaCa-2 cells that pretreated with RSL3 (2 uM) or not. E. Confocal microscopy showed the non-oxidized lipid (red) and oxidized lipid (green) in ARF6 silenced PANC-1 and MIA PaCa-2 cells that pretreated with RSL3 (2 uM) or not.
ARF6 regulates the sensitivity to RSL3 via ACSL4
Since RSL3 directly targets to GPx4, and our results showed that ARF6 affected the severity of lipid peroxidation caused by RSL3. We then tested whether ARF6 regulated GPx4 expression. However, there were no significant changes in the GPx4 protein level after ARF6 knockdown (Figure 3A). Early reports have shown that acyl-CoA synthetase long-chain family member 4 (ACSL4) could determine ferroptosis sensitivity by enriching cellular membranes with long polyunsaturated ω6 fatty acids, which were easy to be oxidized under the condition of oxidative stress. Moreover, ACSL4 could regulated the sensitivity to RSL3-induced ferroptosis [21]. Therefore, we detected ACSL4 protein level by western blotting with ACSL4 antibody and the results showed that ACSL4 protein was increased in ARF6 knockdown cells (Figure 3A). However, ACSL4 mRNA did not show significant changes in ARF6 knockdown cells. We next sought to interrogate whether ARF6 affected the sensitivity to RSL3 via inhibiting ACSL4. Rosiglitazone (ROSI) was reported as a pharmacologically inhibitor of ACSL4. We then used ROSI to inhibit ACSL4 in ARF6 knockdown cells and tested changes in cell viability caused by RSL3. The results demonstrated that ROSI could reverse RSL3 sensitivity caused by ARF6 knockdown (Figure 3C and 3D). Moreover, further results showed that ROSI could reverse RSL3-induced lipid peroxidation in ARF6 knockdown cells (Figure 3E and 3F). These indicated that ARF6 regulated the sensitivity to RSL3-induced ferroptosis via affecting the ACSL4 protein level.
Figure 3.
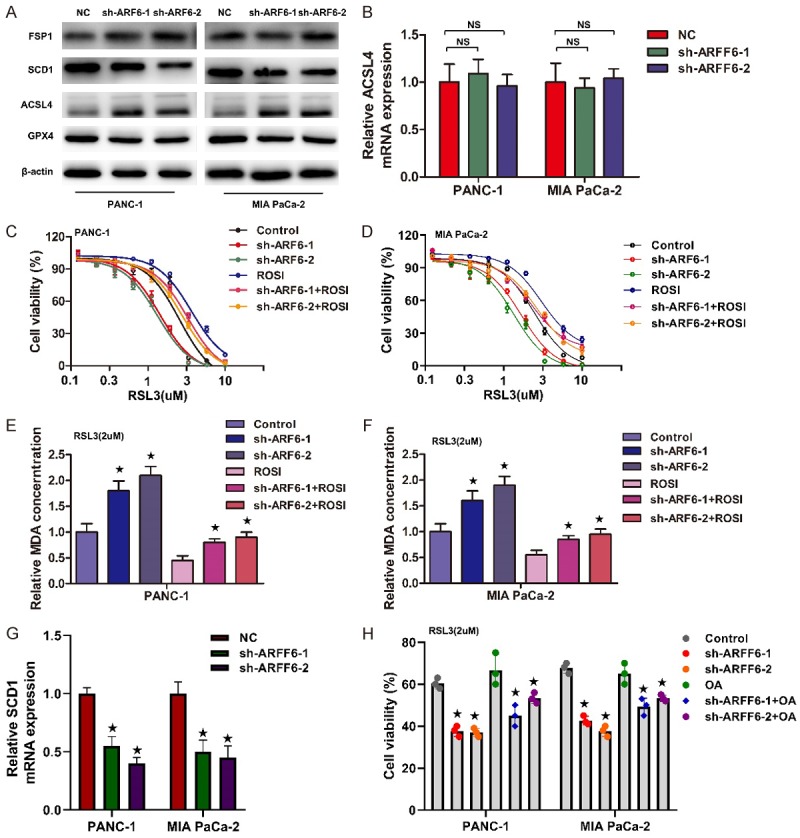
ARF6 regulates the sensitivity to RSL3 via ACSL4. A. Western blotting analyzed ACSL4, GPx4, FSP1, SCD1 expression in PANC-1 and MIA PaCa-2 cells that silenced ARF6 or not. B. qRT-PCR analyzed the change of ACSL4 mRNA in PANC-1 and MIA PaCa-2 cells that silenced ARF6 or not. C and D. Dose-dependent toxicity of RSL3 was analyzed in ARF6 silenced PANC-1 and MIA PaCa-2 that pretreated with ROSI or not. E and F. MDA level was detected in AR6 silenced PANC-1 and MIA PaCa-2 cells that pretreated with ROSI or not. G. qRT-PCR analyzed the change of ACSL4 mRNA in PANC-1 and MIA PaCa-2 cells that silenced ARF6 or not. H. Cell viability was analyzed in ARF6 silenced PANC-1 and MIA PaCa-2 that pretreated with OA (80 mmol/L) or not.
In addition to ACSL4, recent studies also reported that stearoyl-coA desaturase (SCD1) and ferroptosis suppressor protein 1 (FSP1) inhibited the sensitivity to RSL3-induced ferroptosis [22-24]. Therefore, we also detected SCD1 and FSP1 protein level by western blotting and the results showed that SCD1 protein was decreased in ARF6 knockdown cells, while FSP1 did not show significant changes (Figure 3A). Further qRT-PCR showed that SCD1 mRNA also decreased in ARF6 knockdown cells (Figure 3G). However, oleic acid (OA), one of the endproducts of SCD1, could not restore the viability caused by RSL3 in ARF6 knockdown cells (Figure 3H). Therefore, ARF6 might affect RSL3 sensitivity via ACSL4.
ARF6 regulates gemcitabine resistance in pancreatic cancer cells
Our results demonstrated that ARF6 endowed pancreatic cancer cells to a status that is more sensitive to oxidative stress by regulating ACSL4-catalyzed long polyunsaturated ω6 fatty acids. We next sought to explore the significance of this phenomenon. It was validated that ROS promoted the resistance of gemcitabine in pancreatic cancer [25]. However, gemcitabine could also function as an oxidative stress inducer and increased ROS level in pancreatic cancer cells [26]. We sought to further confirm whether gemcitabine could cause lipid oxidation. Intriguingly, with the increase of gemcitabine concentration, the degree of lipid oxidation also increased mildly (Figure 4A). Since ARF6 could regulate RSL3-induced lipid peroxidation, we then test whether ARF6 could regulate gemcitabine-induced lipid peroxidation. Strikingly, ARF6 silenced significantly improved gemcitabine-induced MDA concentration and ROSI could partly counteract this effect (Figure 4B). We further tested gemcitabine sensitivity in PANC-1 and MIA PaCa-2 cell lines. The results revealed that ARF6 silenced markedly mitigated gemcitabine resistance, but ROSI only partly reversed this phenomenon (Figure 4C and 4D). This reminded us that ARF6 might regulate gemcitabine resistance through other pathways. We next tested some gemcitabine resistance related metabolic proteins, RRM1, DCK and hENT1 [27]. Our results showed that ARF6 silenced strikingly increased the level of DCK and hENT1, but there was no significant change in RRM1 protein level (Figure 4E). Further qRT-PCR demonstrated that ARF6 silenced did not affect DCK and hENT1 mRNA level in both PANC-1 and MIA PaCa-2 cell lines (Figure 4F). These might explain why ROSI could not reverse gemcitabine sensitivity caused by ARF6 silenced.
Figure 4.
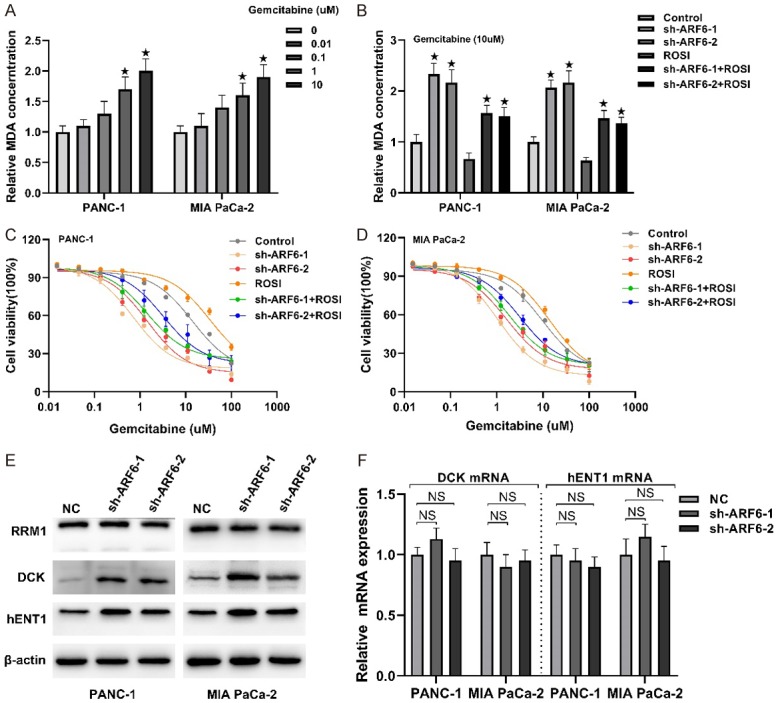
ARF6 regulates gemcitabine resistance in pancreatic cancer cells. A. Gemcitabine was pretreated in PANC-1 and MIA PaCa-2 cells at a series of concentrations for three days, then MDA was detected. B. Gemcitabine (10 uM) was pretreated in ARF6 silenced PANC-1 and MIA PaCa-2 cells that pretreated with ROSI or not. C and D. Dose-dependent toxicity of gemcitabine was analyzed in ARF6 silenced PANC-1 and MIA PaCa-2 that pretreated with ROSI or not. E. Western blotting analyzed the effect of ARF6 on RRM1, DCK and hENT1 protein level in PANC-1 and MIA PaCa-2 cells. F. qRT-PCR analyzed the effect of ARF6 on DCK and hENT1 mRNA level.
ARF6 negatively correlated with ACSL4 expression in pancreatic cancer patients
To validate the expression status between ARF6 and ACSL4 in patients’ tissues, we random selected 40 patients diagnosed with pancreatic cancer in our center. Next, we conducted immunohistochemical staining in their paraffn-embedded tissues with antibodies against ARF6 and ACSL4. Moreover, we calculated IHC scores of ARF6 and ACSL4 by multiplying the proportion scores and intensity scores. The scoring parameters of ARF6 and ACSL4 are listed in Figure 5A and 5B. Then, we conducted statistical analysis of the correlation between ARF6 and ACSL4 and the results showed that there was a significant negative correlation between ARF6 and ACSL4 in pancreatic cancer patients (Figure 5C). Figure 5D showed two typical examples of opposite ARF6 and ACSL4 expressions.
Figure 5.
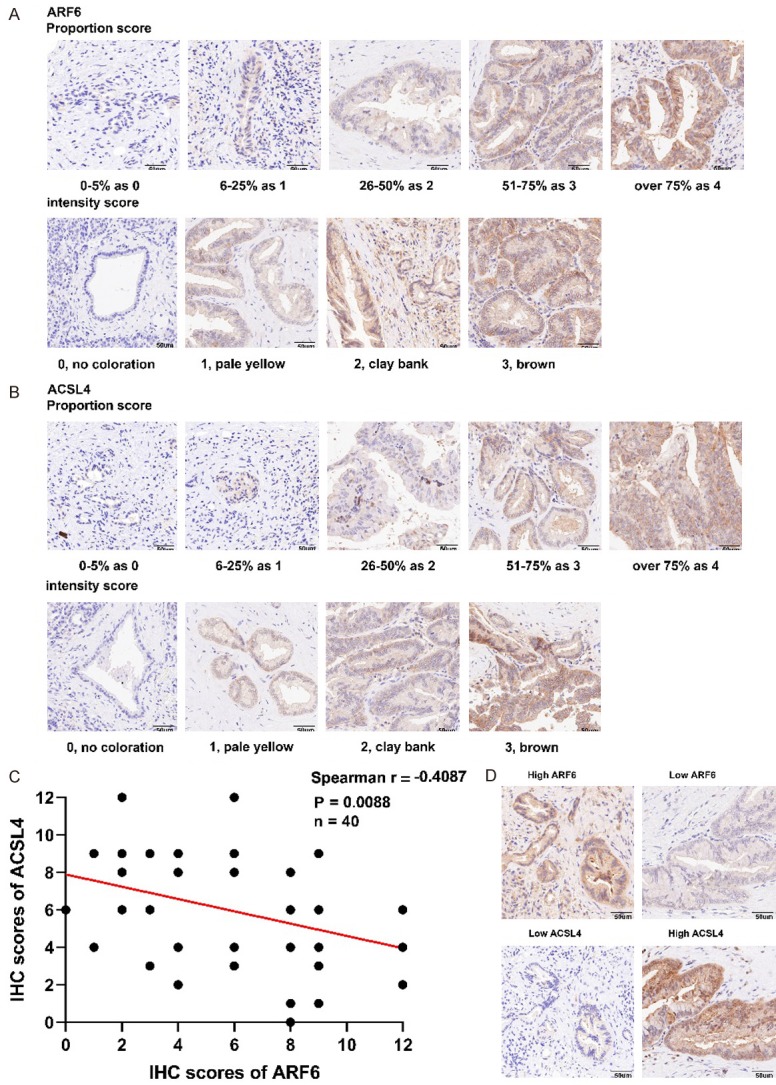
ARF6 negatively correlated with ACSL4 expression in pancreatic cancer patients. A and B. Representative micrographs showing proportion score and intensity score of ARF6 and ACSL4. C. ARF6 negatively correlated with ACSL4 expression in pancreatic cancer patients as demonstrated by immunohistochemical staining and scoring. D. Patients with higher levels of ARF6 displayed lower ACSL4 expression.
Discussion
Ras superfamily play an important role in the physiological activity of cells, and their mutations or abnormal activation will promote cancer development [28,29]. Generally, Ras superfamily can be divided into five main families upon sequence identity and function: Ras, Rho, Rab, Arf and Ran [30]. RAS (KRAS, HRAS, NRAS) is the most commonly mutated oncogene in human cancer, with particularly high frequency in cancers of the pancreas, colon, and lung. Moreover, RAS mutation is associated with poor prognosis and resistance to therapy, yet there are no effective therapies to specifically treat cancers expressing mutant forms of the RAS oncoprotein [31]. Therefore, it is a high priority to find compounds that are selectively lethal to RAS-mutant tumor cells. Brent R. Stockwell screened tens of thousands small compounds to identify specific compounds that could kill RAS mutant cancer cells. They referred to these selected compounds as RAS-selective lethal (RSL) compounds, such as erastin and RSL3 and further revealed the underlying mechanisms. Moreover, they found these compounds killed cells in a new manner that is iron-dependent and different from apoptosis [3,4]. In 2012, they further explored this type of cell death and named it as ferroptosis, which was characterized by iron dependence and lipid peroxidation [32]. Erastin and RSL3 are canonical ferroptosis inducer. Mechanistically, erastin inhibits Na+-independent cystine/glutamate antiporter (system xc -), while RSL3 targets to GPx4 [33].
ARF6, a number of RAS superfamily, exerts an important and complicated effect in cells. But the relationship between ARF6 and ferroptosis is unclear. In this study, we detected that ARF6 silenced sensitized RSL3-induced ferroptosis, not erastin-induced ferroptosis. The underlying mechanism is not clear. Maybe there was activated transsulfuration pathway in pancreatic cancer that could antagonize erastin-induced cystine deprivation [34]. But further study is needed to verified this. We next revealed that ARF6 silenced did not affect the lipid peroxidation level, which was the most important feature of ferroptosis. Intriguingly, ARF6 silenced enhanced RSL3-induced peroxidation. Further study revealed that ARF6 silenced upregulated ACSL4 protein level but not mRNA level. These indicated that ARF6 could regulate ACSL4 in post-translational level. ACSL4 has been verified to dictate the sensitivity of erastin-, RSL3-, or GPx4-depletion-induced ferroptosis by shaping cellular lipid composition to a status that is easy to be oxidized [21,35,36].
Ferroptosis has a broad clinical application prospect. For instance, it has been confirmed that drug-tolerant persisted cancer cells were vulnerable to ferroptosis [37]. In this case, many studies focused on the using nanomaterials as drug carriers to deliver ferroptosis inducers to kill cancer cells in vivo [38]. Moreover, inhibiting or activating ferroptosis regulated the sensitivity to cisplatin [39]. We then explored whether there was a connection between ARF6-regulated ferroptosis and gemcitabine. We found that ARF6 silenced did enhance the sensitivity to gemcitabine in pancreatic cancer, but this effect was not entirely caused by the activation of ferroptosis. Gemcitabine related metabolic proteins, DCK and hENT1, might also involve in this results, because ARF6 silenced also upregulated the protein level of DCK and hENT1.
The limitation of this study was that we did not further illustrate how ARF6 regulated ACSL4 in mechanism. ARF6 has been confirmed that ARF6 localized to the plasma membrane (PM) and vesicle membrane in cytoplasm, where it performed functions in internalization of ligands and recycling endosomes, regulated endocytic membrane trafficking and actin remodeling, promoted autophagy and so on [40,41]. Since ACSL4 was enriched in plasma membrane, we guessed that ARF6 silenced might inhibit the degradation by decreasing the transport to lysosome, for ARF6 has been reported to determine the destination of the cargo in endsome: to be recycled or to be degraded by lysosome [13].
In conclusion, this study revealed the role of ARF6 in RSL3-induced ferroptosis and gemcitabine resistance in pancreatic cancer cells, and preliminarily explored the underlying mechanism (Figure 6).
Figure 6.
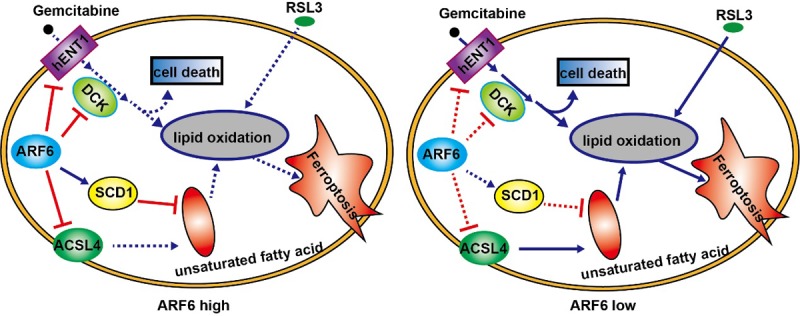
Graphical abstract depicted the function of ARF6 as a suppressor of RSL3-induced ferroptosis by inhibiting ACSL4 and SCD1. ARF6 knockdown endowed cells to a status that sensitized to oxidative stress and then regulated the sensitivity to RSL3. ARF6 also inhibited DCK and hENT1 to regulated gemcitabine resistance.
Acknowledgements
This work was supported by the National Science Foundation of China (grant number 81871950, 81702871 and 81602085).
Disclosure of conflict of interest
None.
References
- 1.Linkermann A, Skouta R, Himmerkus N, Mulay SR, Dewitz C, De Zen F, Prokai A, Zuchtriegel G, Krombach F, Welz PS, Weinlich R, Vanden Berghe T, Vandenabeele P, Pasparakis M, Bleich M, Weinberg JM, Reichel CA, Brasen JH, Kunzendorf U, Anders HJ, Stockwell BR, Green DR, Krautwald S. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111:16836–16841. doi: 10.1073/pnas.1415518111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devos D, Moreau C, Devedjian JC, Kluza J, Petrault M, Laloux C, Jonneaux A, Ryckewaert G, Garcon G, Rouaix N, Duhamel A, Jissendi P, Dujardin K, Auger F, Ravasi L, Hopes L, Grolez G, Firdaus W, Sablonniere B, Strubi-Vuillaume I, Zahr N, Destee A, Corvol JC, Poltl D, Leist M, Rose C, Defebvre L, Marchetti P, Cabantchik ZI, Bordet R. Targeting chelatable iron as a therapeutic modality in Parkinson’s disease. Antioxid Redox Signal. 2014;21:195–210. doi: 10.1089/ars.2013.5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 4.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bebber CM, Muller F, Prieto Clemente L, Weber J, von Karstedt S. Ferroptosis in cancer cell biology. Cancers (Basel) 2020;12 doi: 10.3390/cancers12010164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shan X, Li S, Sun B, Chen Q, Sun J, He Z, Luo C. Ferroptosis-driven nanotherapeutics for cancer treatment. J Control Release. 2020;319:322–332. doi: 10.1016/j.jconrel.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 7.Roh JL, Kim EH, Jang HJ, Park JY, Shin D. Induction of ferroptotic cell death for overcoming cisplatin resistance of head and neck cancer. Cancer Lett. 2016;381:96–103. doi: 10.1016/j.canlet.2016.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Zhang X, Sui S, Wang L, Li H, Zhang L, Xu S, Zheng X. Inhibition of tumor propellant glutathione peroxidase 4 induces ferroptosis in cancer cells and enhances anticancer effect of cisplatin. J Cell Physiol. 2020;235:3425–3437. doi: 10.1002/jcp.29232. [DOI] [PubMed] [Google Scholar]
- 9.Sato M, Kusumi R, Hamashima S, Kobayashi S, Sasaki S, Komiyama Y, Izumikawa T, Conrad M, Bannai S, Sato H. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci Rep. 2018;8:968. doi: 10.1038/s41598-018-19213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–9705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heinemann V. Gemcitabine: progress in the treatment of pancreatic cancer. Oncology. 2001;60:8–18. doi: 10.1159/000055290. [DOI] [PubMed] [Google Scholar]
- 12.Makohon-Moore A, Iacobuzio-Donahue CA. Pancreatic cancer biology and genetics from an evolutionary perspective. Nat Rev Cancer. 2016;16:553–565. doi: 10.1038/nrc.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Acker T, Tavernier J, Peelman F. The small GTPase Arf6: an overview of its mechanisms of action and of its role in host(-)pathogen interactions and innate immunity. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20092209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li R, Peng C, Zhang X, Wu Y, Pan S, Xiao Y. Roles of Arf6 in cancer cell invasion, metastasis and proliferation. Life Sci. 2017;182:80–84. doi: 10.1016/j.lfs.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Montagnac G, de Forges H, Smythe E, Gueudry C, Romao M, Salamero J, Chavrier P. Decoupling of activation and effector binding underlies ARF6 priming of fast endocytic recycling. Curr Biol. 2011;21:574–579. doi: 10.1016/j.cub.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 16.Radhakrishna H, Donaldson JG. ADP-ribosylation factor 6 regulates a novel plasma membrane recycling pathway. J Cell Biol. 1997;139:49–61. doi: 10.1083/jcb.139.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu W, Liu J, Xiang J, Liang D, Hu Q, Ni Q, Yu X, Xu J. ARF6, induced by mutant Kras, promotes proliferation and Warburg effect in pancreatic cancer. Cancer Lett. 2017;388:303–311. doi: 10.1016/j.canlet.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Y, Mao C, Yang R, Yan B, Shi Y, Liu X, Lai W, Liu Y, Wang X, Xiao D, Zhou H, Cheng Y, Yu F, Cao Y, Liu S, Yan Q, Tao Y. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7:3293–3305. doi: 10.7150/thno.19988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ji S, Qin Y, Liang C, Huang R, Shi S, Liu J, Jin K, Liang D, Xu W, Zhang B, Liu L, Liu C, Xu J, Ni Q, Chiao PJ, Li M, Yu X. FBW7 (F-box and WD repeat domain-containing 7) negatively regulates glucose metabolism by targeting the c-Myc/TXNIP (thioredoxin-binding protein) axis in pancreatic cancer. Clin Cancer Res. 2016;22:3950–3960. doi: 10.1158/1078-0432.CCR-15-2380. [DOI] [PubMed] [Google Scholar]
- 20.Hu Q, Qin Y, Ji S, Xu W, Liu W, Sun Q, Zhang Z, Liu M, Ni Q, Yu X, Xu X. UHRF1 promotes aerobic glycolysis and proliferation via suppression of SIRT4 in pancreatic cancer. Cancer Lett. 2019;452:226–236. doi: 10.1016/j.canlet.2019.03.024. [DOI] [PubMed] [Google Scholar]
- 21.Doll S, Proneth B, Tyurina YY, Panzilius E, Kobayashi S, Ingold I, Irmler M, Beckers J, Aichler M, Walch A, Prokisch H, Trumbach D, Mao G, Qu F, Bayir H, Fullekrug J, Scheel CH, Wurst W, Schick JA, Kagan VE, Angeli JP, Conrad M. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13:91–98. doi: 10.1038/nchembio.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tesfay L, Paul BT, Konstorum A, Deng Z, Cox AO, Lee J, Furdui CM, Hegde P, Torti FM, Torti SV. Stearoyl-CoA desaturase 1 protects ovarian cancer cells from ferroptotic cell death. Cancer Res. 2019;79:5355–5366. doi: 10.1158/0008-5472.CAN-19-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doll S, Freitas FP, Shah R, Aldrovandi M, da Silva MC, Ingold I, Grocin AG, Xavier da Silva TN, Panzilius E, Scheel CH, Mourao A, Buday K, Sato M, Wanninger J, Vignane T, Mohana V, Rehberg M, Flatley A, Schepers A, Kurz A, White D, Sauer M, Sattler M, Tate EW, Schmitz W, Schulze A, O’Donnell V, Proneth B, Popowicz GM, Pratt DA, Angeli JPF, Conrad M. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575:693–698. doi: 10.1038/s41586-019-1707-0. [DOI] [PubMed] [Google Scholar]
- 24.Bersuker K, Hendricks JM, Li Z, Magtanong L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, Bassik MC, Nomura DK, Dixon SJ, Olzmann JA. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575:688–692. doi: 10.1038/s41586-019-1705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meng Q, Shi S, Liang C, Liang D, Hua J, Zhang B, Xu J, Yu X. Abrogation of glutathione peroxidase-1 drives EMT and chemoresistance in pancreatic cancer by activating ROS-mediated Akt/GSK3beta/Snail signaling. Oncogene. 2018;37:5843–5857. doi: 10.1038/s41388-018-0392-z. [DOI] [PubMed] [Google Scholar]
- 26.Zhang Z, Duan Q, Zhao H, Liu T, Wu H, Shen Q, Wang C, Yin T. Gemcitabine treatment promotes pancreatic cancer stemness through the Nox/ROS/NF-kappaB/STAT3 signaling cascade. Cancer Lett. 2016;382:53–63. doi: 10.1016/j.canlet.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 27.de Sousa Cavalcante L, Monteiro G. Gemcitabine: metabolism and molecular mechanisms of action, sensitivity and chemoresistance in pancreatic cancer. Eur J Pharmacol. 2014;741:8–16. doi: 10.1016/j.ejphar.2014.07.041. [DOI] [PubMed] [Google Scholar]
- 28.Colicelli J. Human RAS superfamily proteins and related GTPases. Sci STKE. 2004;2004:RE13. doi: 10.1126/stke.2502004re13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wennerberg K, Rossman KL, Der CJ. The ras superfamily at a glance. J Cell Sci. 2005;118:843–846. doi: 10.1242/jcs.01660. [DOI] [PubMed] [Google Scholar]
- 30.Vigil D, Cherfils J, Rossman KL, Der CJ. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat Rev Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Haigis KM. KRAS alleles: the devil is in the detail. Trends Cancer. 2017;3:686–697. doi: 10.1016/j.trecan.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B 3rd, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayano M, Yang WS, Corn CK, Pagano NC, Stockwell BR. Loss of cysteinyl-tRNA synthetase (CARS) induces the transsulfuration pathway and inhibits ferroptosis induced by cystine deprivation. Cell Death Differ. 2016;23:270–278. doi: 10.1038/cdd.2015.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan H, Li X, Zhang X, Kang R, Tang D. Identification of ACSL4 as a biomarker and contributor of ferroptosis. Biochem Biophys Res Commun. 2016;478:1338–1343. doi: 10.1016/j.bbrc.2016.08.124. [DOI] [PubMed] [Google Scholar]
- 36.Kagan VE, Mao G, Qu F, Angeli JP, Doll S, Croix CS, Dar HH, Liu B, Tyurin VA, Ritov VB, Kapralov AA, Amoscato AA, Jiang J, Anthonymuthu T, Mohammadyani D, Yang Q, Proneth B, Klein-Seetharaman J, Watkins S, Bahar I, Greenberger J, Mallampalli RK, Stockwell BR, Tyurina YY, Conrad M, Bayir H. Oxidized arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem Biol. 2017;13:81–90. doi: 10.1038/nchembio.2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hangauer MJ, Viswanathan VS, Ryan MJ, Bole D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL, McCormick F, McManus MT. Drug-tolerant persister cancer cells are vulnerable to GPX4 inhibition. Nature. 2017;551:247–250. doi: 10.1038/nature24297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30:e1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Wang K. The induction of ferroptosis by impairing STAT3/Nrf2/GPx4 signaling enhances the sensitivity of osteosarcoma cells to cisplatin. Cell Biol Int. 2019;43:1245–1256. doi: 10.1002/cbin.11121. [DOI] [PubMed] [Google Scholar]
- 40.Wolff NA, Lee WK, Abouhamed M, Thevenod F. Role of ARF6 in internalization of metal-binding proteins, metallothionein and transferrin, and cadmium-metallothionein toxicity in kidney proximal tubule cells. Toxicol Appl Pharmacol. 2008;230:78–85. doi: 10.1016/j.taap.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 41.Moreau K, Rubinsztein DC. The plasma membrane as a control center for autophagy. Autophagy. 2012;8:861–863. doi: 10.4161/auto.20060. [DOI] [PMC free article] [PubMed] [Google Scholar]