Abstract
We report the case of a 71-year-old Japanese man with a history of chronic kidney disease and sarcoidosis receiving chronic corticosteroids who presented with disseminated Nocardia brasiliensis infection. He initially showed improvement with empiric antimicrobial therapy including trimethoprim-sulfamethoxazole. However, he deteriorated after modifying the empiric regimen due to complicated hyperkalemia and ultimately died. In general, elderly patients have decreased renal function. Standard therapy for nocardiosis with trimethoprim-sulfamethoxazole may not be used for a prolonged period of time. This case emphasizes the challenges and importance of prudent selection of empiric antimicrobial therapy for disseminated nocardiosis in elderly patients with underlying kidney disease.
Keywords: disseminated nocardiosis, Nocardia brasiliensis, trimethoprim-sulfamethoxazole, linezolid
Introduction
Nocardiosis is a major opportunistic bacterial infection caused by aerobic, gram-positive bacillus in the genus Nocardia [[1], [2], [3], [4]]. Disseminated or systemic nocardiosis is a life-threatening infection involving > 2 organ sites, such as the skin, lungs, and central nervous system [2,5]. Early detection and effective antimicrobial treatment are essential to achieve favorable outcome. Trimethoprim-sulfamethoxazole (TMP-SMX) is a critical drug of choice for severe nocardiosis in combination with other antimicrobial agents. Although TMP-SMX is well tolerated in many patients, clinicians should be aware of possible adverse effects, such as electrolyte disturbances, renal dysfunction, dermatologic reactions, and hypersensitivity. Consequently, some patients with nocardiosis need to withhold the medication despite the risk of treatment failure.
Here, we report an elderly patient who presented with disseminated nocardiosis caused by Nocardia brasiliensis. The antimicrobial therapy for this patient needed to be modified due to renal impairment and hyperkalemia, which subsequently led to ineffective empiric treatment against N. brasiliensis. This report highlights the pitfalls in treating disseminated nocardiosis in elderly patients, especially those with underlying kidney disease.
Case Presentation
A 71-year-old Japanese man with a history of chronic kidney disease and sarcoidosis with prolonged use of oral prednisolone presented to our emergency department with fever, skin rash, and dyspnea. Two weeks earlier, he developed a fever of 38 °C, which improved spontaneously after a few days. The fever recurred, however, with rashes on the forehead and left earlobe 1 week prior to admission. He had become progressively lethargic and developed confusion. He also reported exertional dyspnea and left leg pain.
The patient was diagnosed with sarcoidosis based on thoracoscopic lymph node biopsy approximately 17 years prior to admission. In the 2 years before admission, his sarcoidosis was treated with oral prednisolone 15 mg/day for chronic cough and worsening lung infiltrates. His other medical history included hypertension and chronic kidney disease, for which he received amlodipine and telmisartan. He had received no prophylaxis for pneumocystis pneumonia. His hobby was gardening and he grew several vegetables in his garden.
On admission, his temperature was 38.1 °C, blood pressure was 144/81 mmHg, heart rate was 133 beats/min, respiratory rate was 30 breaths/min, and oxygen saturation was 69% on ambient air. He appeared confused and severely ill. On physical examination, breath sounds were harsh and coarse crackles were heard at the middle and lower areas of both lungs. Dermatologic examination revealed various skin lesions, including erythema with crusting, ulcerations, vesicles, and pustules on the forehead, neck, left earlobe, and extremities (Fig. 1). Results of laboratory examination and arterial blood gas analysis on admission are presented in Table 1. Two sets of blood cultures were obtained. Chest radiography revealed bilateral diffuse patches and nodular opacities. Plain computed tomography (CT) of the chest demonstrated multiple peripheral lung nodules with bilateral pleural effusion, emphysematous changes, and cavitation at the right upper lobe (Fig. 2).
Fig. 1.
Skin lesions on admission. (A) Erythema with crusting and ulcerations (red arrow) on the forehead. (B) Pustules (black arrow) on the neck. (C) Vesicles (black arrowhead) on the right ankle.
Table 1.
Laboratory Data
| Variables | On Admission | The 5th day of admission | The 9th day of admission |
|---|---|---|---|
| Blood | |||
| White cell count (per mm3) | 8,400 | 7,400 | 17,200 |
| Hemoglobin (g/dL) | 12.0 | 7.2 | 8.2 |
| Platelet (per mm3) | 387,000 | 135,000 | 307,000 |
| Albumin (g/dL) | 1.6 | 1.1 | |
| Blood urea nitrogen (mg/dL) | 58 | 73 | 66 |
| Creatinine (mg/dL) | 1.80 | 1.29 | 1.11 |
| Sodium (mEq/L) | 149 | 145 | 146 |
| Potassium (mEq/L) | 4.9 | 5.4 | 6.0 |
| Chloride (mEq/L) | 110 | 115 | 121 |
| Arterial Blood Gas (Ambient air) | |||
| pH | 7.402 | ||
| pCO2 (mmHg) | 42.0 | ||
| pO2 (mmHg) | 41.6 | ||
| Bicarbonate (mmol/L) | 25.5 |
Fig. 2.
Plain computed tomography findings. (A) Multiple peripheral lung nodules with bilateral pleural effusion on admission. (B) Cavitation at the right upper lobe on admission. (C) Worsening cavitation on day 10.
During the initial evaluation, the patient quickly deteriorated with severe respiratory distress and hypotension. He was intubated and transferred to the intensive care unit with airborne isolation. Given his severe condition requiring intensive care, immunocompromised status, and current influenza epidemic season, we administered piperacillin-tazobactam, minocycline, vancomycin, and peramivir as empiric therapy. TMP-SMX was also administered to treat possible pneumocystis pneumonia and Nocardia infection with a renal-adjusted dose of 80 mg trimethoprim 3 times/day orally (5 mg/kg/day) through a nasogastric tube. The dose of prednisolone was increased to 60 mg/day for possible exacerbation of sarcoidosis. Bronchoscopy was performed and the differential bronchoalveolar lavage fluid (BALF) cell counts were predominately lymphocytes (64%). Gram, Grocott, and Ziehl-Neelsen staining of the BALF showed no microorganisms. Acyclovir was added as empiric therapy for disseminated varicella-zoster virus infection after consultation with the Department of Dermatology for his multiple skin lesions.
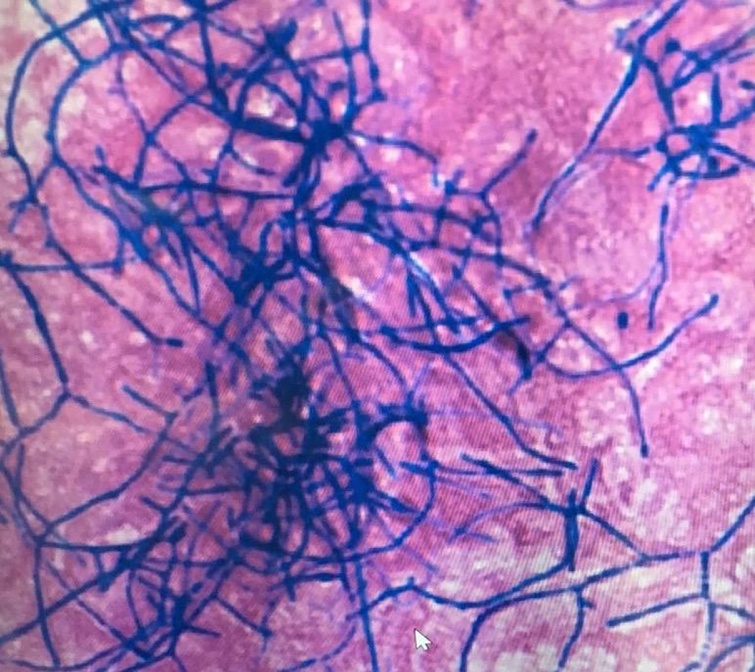
On the fifth day of admission, the BALF culture grew thin filamentous branching gram-positive bacteria with acid-fast–positive staining (Fig. 3). Shortly afterward, histopathologic examination of the biopsy specimens of the skin lesions on the neck and left ankle revealed similar gram-positive filamentous rods. Disseminated nocardiosis was clinically diagnosed, and piperacillin-tazobactam was switched to cefepime. Polymerase chain reaction testing of the BALF revealed positive results for Pneumocystis jirovecii. Thus, with a diagnosis of coinfection with P. jirovecii, the dose of TMP-SMX was increased to 240 mg trimethoprim 2 times/day orally (10 mg/kg/day). Vancomycin and acyclovir were discontinued. His serum potassium level gradually increased to 6.0 mEq/L on the ninth day despite potassium-lowering treatment, whereas his serum creatinine level improved to 1.1 mg/dL (Table 1). TMP-SMX was discontinued to control his hyperkalemia, and atovaquone was added to treat pneumocystis pneumonia.
Fig. 3.
Culture from bronchoalveolar lavage sample. Thin filamentous branching gram-positive bacteria were observed with Gram staining.
On the following day, he deteriorated with desaturation. Plain CT of the chest demonstrated diffuse infiltrates, increased pleural effusion, and worsening of the cavitary lesion at the right upper lobe (Fig. 2). Repeat bronchoscopy showed no new findings. Cefepime and minocycline were replaced by meropenem in order to improve the coverage of Nocardia species and gram-negative bacilli. Voriconazole and ganciclovir were also administered as empiric antimicrobial therapy for invasive aspergillosis and cytomegalovirus infection, respectively.
On the 14th day of admission, he developed hypotension and altered mental status. Transthoracic cardiac ultrasound revealed vegetation at the mitral valve, and vancomycin was restarted to treat infective endocarditis. On the same day, the organism isolated from the BALF culture was identified as N. brasiliensis by standard biochemical test. The isolate was determined to be resistant to meropenem by the automated MicroScan WalkAway System with Pos combo 3.1 J panel (Beckman Coulter, Brea, CA, USA) (Table 2). Thus, TMP-SMX and minocycline were resumed. Repeat contrast-enhanced CT of the chest, abdomen, and pelvis revealed worsening lung infiltrates, bilateral pleural effusion, and subcutaneous abscesses in the left thigh. CT scan of the head without contrast material showed no abnormalities consistent with nocardiosis. He developed multiorgan failure, and continuous hemodiafiltration was initiated. Considering his progressive clinical course, palliative care was provided by the multidisciplinary intensive care team with infectious disease specialists and a nephrologist. He died on the 24th day of admission.
Table 2.
Antimicrobial susceptibility testing results
| Antimicrobial Agents | Susceptibility |
|---|---|
| Ceftriaxone | Resistant |
| Imipenem-cilastatin | Resistant |
| Amikacin | Susceptible |
| Minocycline | Susceptible |
| Trimethoprim-sulfamethoxazole | Susceptible |
| Linezolid | Susceptible |
Consent Section: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Discussion
We reported the case of a 71-year-old Japanese man with a history of pulmonary sarcoidosis and prolonged immunosuppressant use who presented with fever, skin rash, and dyspnea, and was later diagnosed with disseminated N. brasiliensis infection. Antimicrobial selection of meropenem monotherapy, as a result of severe hyperkalemia due to TMP-SMX, turned out to be ineffective against N. brasiliensis. N. brasiliensis is highly susceptible to TMP-SMX and amikacin, whereas imipenem and meropenem tend to be less active [6,7].
Disseminated or systemic nocardiosis is a life-threatening infection. A definitive diagnosis usually depends on the demonstration of the organism from a clinical specimen [4]. Growth of Nocardia species in routine aerobic cultures usually requires prolonged incubation (up to two weeks) [3]. Due to the nonspecific and diverse presentation of nocardiosis, the clinician should consider a broad differential diagnosis. In this case, we considered the differential diagnosis include Pneumocystis pneumonia, disseminated herpes simplex virus infection, Mycobacterium, and fungal infections.
The treatment of disseminated nocardiosis requires empiric combination therapy due to the high variability of antimicrobial susceptibility testing results among the species of Nocardia. Most experts recommend TMP-SMX as the drug of choice, although previous reports indicated their incidence of resistance cannot be negligible [[2], [3], [4]]. Amikacin and imipenem are also widely used in combination of TMP-SMX [8]. However, clinicians should be cognizant of possible adverse reactions to TMP-SMX [9,10]. Hyperkalemia is a relatively common and potentially fatal adverse reaction to TMP-SMX, especially in older patients with underlying kidney disease. In addition, amikacin has a risk of nephrotoxicity, which is problematic when using this agent in patients with renal insufficiency.
Recent studies have demonstrated linezolid as a second-line treatment option for disseminated nocardiosis [11]. Linezolid has no risk of nephrotoxicity and can be safely used in patients with renal insufficiency [12]. However, long-term use of linezolid is associated with significant risk of myelosuppression, which should be monitored with caution [13].
Conclusion
Empiric therapy for disseminated nocardiosis should include multiple antimicrobial agents. TMP-SMX is the most reliable drug of choice for disseminated nocardiosis. However, some elderly patients cannot tolerate this medication, which can pose a real challenge for clinicians, and potentially be life threatening. Nonetheless, linezolid can be an effective short-term alternative therapy for disseminated nocardiosis in such situations.
Author Statement
K.N., H.I., H.G., K.H. designed the study and wrote the manuscript. S.T. N.T contributed to collection and analysis of data. All authors critically reviewed the manuscript and approved the final version of the manuscript.
Declaration of Competing Interest
The authors declare no conflicts of interest.
References
- 1.Brown-Elliott B.A., Brown J.M., Conville P.S., Wallace R.J. Clinical and Laboratory Features of the Nocardia spp. Based on Current Molecular Taxonomy. Clin Microbiol Rev. 2006;19:259–282. doi: 10.1128/CMR.19.2.259-282.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wilson J.W. Nocardiosis: Updates and Clinical Overview. Jmcp. 2012;87:403–407. doi: 10.1016/j.mayocp.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beaman B.L., Beaman L. Nocardia species: host-parasite relationships. Clin Microbiol Rev. 1994;7:213–264. doi: 10.1128/cmr.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.John E.B., Raphael D., Martin J.B. 8th ed. Elsevier/Saunders; Philadelphia: 2015. Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases; pp. 2853–2863. [Google Scholar]
- 5.McNeil M.M., Brown J.M. The medically important aerobic actinomycetes: epidemiology and microbiology. Clin Microbiol Rev. 1994;7:357–417. doi: 10.1128/cmr.7.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown-Elliott B.A., Killingley J., Vasireddy S., Bridge L., Wallace R.J., Jr In VitroComparison of Ertapenem, Meropenem, and Imipenem against Isolates of Rapidly Growing Mycobacteria and Nocardia by Use of Broth Microdilution and Etest. J Clin Microbiol. 2016;54:1586–1592. doi: 10.1128/JCM.00298-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McTaggart L.R., Doucet J., Witkowska M., Richardson S.E. Antimicrobial Susceptibility among Clinical Nocardia Species Identified by Multilocus Sequence Analysis. Antimicrob Agents Chemother. 2014;59:269–275. doi: 10.1128/AAC.02770-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Restrepo A., Clark N.M. on behalf of the Infectious Diseases Community of Practice of the American Society of Transplantation. Nocardia infections in solid organ transplantation: Guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation. Clinical Transplantation. 2019;33 doi: 10.1111/ctr.13509. 2853–12. [DOI] [PubMed] [Google Scholar]
- 9.Ho J.M.-W., Juurlink D.N. Considerations when prescribing trimethoprim-sulfamethoxazole. Cmaj. 2011;183:1851–1858. doi: 10.1503/cmaj.111152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alappan R., Perazella M.A., Buller G.K. Hyperkalemia in hospitalized patients treated with trimethoprim-sulfamethoxazole. Ann Intern Med. 1996;124:316–320. doi: 10.7326/0003-4819-124-3-199602010-00006. [DOI] [PubMed] [Google Scholar]
- 11.La Cruz De O., Minces L.R., Silveira F.P. Experience with linezolid for the treatment of nocardiosis in organ transplant recipients. Journal of Infection. 2015;70:44–51. doi: 10.1016/j.jinf.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Brier M.E., Stalker D.J., Aronoff G.R., Batts D.H., Ryan K.K., O’Grady M. Pharmacokinetics of Linezolid in Subjects with Renal Dysfunction. Antimicrob Agents Chemother. 2003;47:2775–2780. doi: 10.1128/AAC.47.9.2775-2780.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bishop E., Melvani S., Howden B.P., Charles P.G.P., Grayson M.L. Good Clinical Outcomes but High Rates of Adverse Reactions during Linezolid Therapy for Serious Infections: a Proposed Protocol for Monitoring Therapy in Complex Patients. Antimicrob Agents Chemother. 2006;50:1599–1602. doi: 10.1128/AAC.50.4.1599-1602.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]