Abstract
There is no ethnobotanical study conducted specifically on medicinal plants traditionally used to treat cancer in Ethiopia. Yet, traditional herbalists in different parts of the country claim that they have been treating cancer-like symptoms using herbal remedies. The objective of this study was to document medicinal plants traditionally used to treat cancer-like symptoms in eleven districts, Ethiopia. Traditional herbalists were interviewed using semistructured questionnaires, and field visits were also carried out to collect claimed plants for identification purpose. Seventy-four traditional herbalists, who claimed that they knew about and/or had used medicinal plants to treat cancer-like symptoms, were selected using the snowball method and interviewed. Herbalists used their intuition and relied on the chronicity, growth of external mass, and spreading of the disease to other parts of the body, as a means to characterize cancer symptoms. Furthermore, in some of the study districts, herbalists reported that they treat patients who had already been diagnosed in modern healthcare institutions prior to seeking help from them. The inventory of medicinal plants is summarized in a synoptic table, which contains the scientific and vernacular names of the plants, their geographical location, the parts of the plants, and the methods used to prepare the remedies. A total of 53 traditionally used anticancer plants, belonging to 30 families, were identified during the survey. The most frequently reported anticancer plants were Acmella caulirhiza Del (Asteraceae), Clematis simensis Fresen. (Ranunculaceae), Croton macrostachyus Del. (Euphorbiaceae), and Dorstenia barnimiana Schweinf. (Moraceae). Organizing traditional healers, documenting their indigenous knowledge, and scientifically validating it for the development of better cancer therapeutic agents constitute an urgent and important task for policymakers and scientists.
1. Introduction
Cancer is a complex disease that is very heterogenic and variable at cellular level and also differs from one patient to the other in its behaviour, development, and outcome [1]. Physical, metabolic, and behavioural variations of cancer cells from normal ones arise through the accumulation of genetic modifications and help them to proliferate rapidly, escape from host immune surveillance, and ultimately invade distant tissues [2]. Histopathological, genetic, and epigenetic and clinical outcome variations between and within different types of cancers have been the greatest challenge to understand the disease and develop novel therapies [3].
Surgery and radiation therapy were the most preferred means of treatment to control cancer before 1950 and after 1960, respectively [4]. Chemotherapy can be done before surgery to shrink the tumor or after surgery to kill the remaining cancer cells [5]. However, most of the chemotherapeutic drugs lack specificity and tend to rapidly damage normal dividing tissues, causing side effects such as immunosuppression, neurotoxicity, and hair loss [6]. Moreover, resistance has also reduced therapeutic efficacy of some anticancer chemotherapeutic drugs [7].
In order to address these limitations, tapping nature as a major source of chemically diverse novel anticancer compounds is a consistently proven track [8]. Screening natural products yield more hit with more “drug-like” characteristics (absorption and metabolism) as compared to screening of rationally designed compounds [9]. Furthermore, screening medicinal plants based on traditional use provides a higher chance of finding active plants relative to the random approach [10].
Ethiopia has a rich and diverse heritage of traditional medical practices, known for using plants to prepare more than 90% of the remedies [11]. In addition, the country has more than 6,500 higher plant species of which, around 12% are endemic [12]. Reports indicate that up to 80% of the population relies on traditional remedies as a primary source of health care [13]. Only few ethnobotanical reports from different agroecological zones of Ethiopia are available in the literature regarding medicinal plants used for cancer treatment. These include Bersama abyssinica, Buddleja polystachya, Clerodendrum myricoides, Dovyalis abyssinica, Ekebergia capensis, Myrsine melanophloeos, Olea capensis, Pentas lanceolata, Sideroxylon oxyacanthum, and Zingiber officinale [14]; Bidens macroptera, Clematis simensis, Ferula communis, and Punica granatum [15]; Rumex abyssinicus [16]; Zanthoxylum chalybeum [17]; Phytolacca dodecandra and Vinca rosea [18]; Kalanchoe lanceolata, Stephania abyssinica, and Vernonia hymenolepis [19]; Plumbago zeylanica [20–22]; Acalypha acrogyna, Carissa spinarum, Maytenus ovatus, and Salvia nilotica [23]; Croton macrostachyus [24]; and Dorstenia barnimiana [25, 26].
In view of this fact and considering the weak traditional recording and knowledge transfer system and an alarming rate of environmental degradation, finding anticancer plants and documenting their ethnobotanical information constitute an urgent and indispensable task. Therefore, the main aim of this study was to establish an inventory of medicinal plants traditionally used to treat cancer in eleven districts of Ethiopia.
2. Materials and Methods
2.1. Description of the Study Areas
This ethnobotanical study was conducted in four national regional states of Ethiopia: Oromia, Amhara, Afar, and Southern Nations, Nationalities, and People. The survey included different districts from each region, namely, Bale Robe and Goba from Oromia, Bahir Dar Zuria and Filiklik from Amhara, Gewane from Afar, and Wondo Genet, Sodo Zuria, Doyo Gena, North Bench, Mizan Aman, and Shako from Southern Nations, Nationalities, and People Regional State (Figure 1). These geographically, culturally, and agroecologically different study areas (Table 1) were selected mainly based on the availability of traditional healers and recommendations from health workers.
Figure 1.
Map of Ethiopia showing the location of study districts.
Table 1.
Vegetation type, climatic condition, and demographic data of the study areas [27, 28] (source: National Meteorological Service Agency of Ethiopia).
| District | Distance from capital city (km) | Approximate population (2015) | Number of interviewed healers | Area size (km2) | Geographical location | Average elevation above sea level (m.a.s.l) | Vegetation type | Climatic condition (2014) | |
|---|---|---|---|---|---|---|---|---|---|
| Annual average rainfall (mm) | Annual average temperature range (°C) | ||||||||
| Bale Robe | 432 | 65,284 | 2 | 8.87 | 7°07′11.65″ N 40°00′24.82″ E | 2480 | DAF | 745.6 | 9.2–23.2 |
| Goba | 444 | 47,135 | 7 | 20.15 | 7°00′41.66″ N 39°58′33.96″ E | 2614 | DAF | 736.3 | 9.5–23.8 |
| Bahir Dar Zuria | 578 | 206,708 | 16 | 1443.37 | 11°34′27.15″ N 37°21′40.87″ E | 1800 | CTW, DAF, and FLV/MFS | 1547.1 | 12.7–27.6 |
| Filiklik | 188 | 142,722 | 7 | 806.98 | 10°02′12.74″ N 38°14′27.65″ E | 1853 | CTW and DAF | 880.2 | 12.9–22.0 |
| Gewane | 344 | 39,186 | 6 | 967.85 | 10°29′59.99″ N 40°44′59.99″ E | 568 | ACB | 586.7 | 19.5–36.7 |
| Wondo Genet | 270 | 196,277 | 12 | 226.45 | 7°05′3.01″ N 38°37′8.02″ E | 1742 | DAF | 928.7 | 15–29.6 |
| Sodo Zuria | 383 | 145,092 | 2 | 25.62 | 6°51′10.11″ N 37°45′39.49″ E | 1854 | CTW and DAF | 1569.2 | 14.8–25.2 |
| Doyo Gena | 258 | 95,393 | 14 | 130.57 | 7°21′20.22″ N 37°47′07.15″ E | 2300 | DAF | 1334.5 | 11–22.8 |
| North Bench | 587 | 126,308 | 4 | 392.65 | 6°37′53.43″ N 35°33′56.83″ E | 2367 | CTW | 1671.8 | 16–33.3 |
| Mizan Aman | 565 | 64,996 | 3 | 24.45 | 6°59′37.13″ N 35°34′55.92″ E | 1441 | CTW and MAF | 1963.7 | 14.8–28.8 |
| Shako | 617 | 51,195 | 1 | 48,089.63 ha | 7°33′42.37″ N 35°39′11.83″ E | 1800 | CTW and MAF | 1906.9 | 11.4–22.4 |
Note. Vegetation type: DAF: dry evergreen Afromontane forest and grassland complex; CTW: Combretum-Terminalia woodland and wooded grassland; FLV/MFS: freshwater marshes and swamps, floodplains, and lake shore vegetation; ACB: Acacia-Commiphora woodland and bushland proper; MAF: moist evergreen Afromontane forest. m.a.s.l: meter above sea level; mm: millimeter; °C: degree Celsius; km2: kilometer square.
2.2. Data Collection
A team comprising a botanist and researchers from Addis Ababa University was set up, and health authorities were contacted for permission and identification of traditional herbalists living in each study area. Altogether, 117 traditional healers were approached using the snowball technique and 74 traditional healers who used herbs to manage cancer-like symptoms were selected. Ethnobotanical data were collected between January and August 2016, mainly through individual interviews with the selected traditional herbalists using a semistructured interview questionnaire. The questionnaire was prepared in Amharic language and translated to different local languages for traditional healers who do not speak Amharic. This questionnaire was designed to obtain information in the following areas: (i) general data on the informant, (ii) school attendance, (iii) use of plants for cancer treatment, (iv) source of the plant material, (v) part of the plant used, (vi) method of medicinal preparation, (vii) route of administration, and (viii) side effects.
A traditional healer for the purpose of this study is “a person who is recognized by the community in which s/he lives as competent to provide healthcare by using plants and plant products.” Each traditional healer was approached, briefed about the purpose of the research, and asked for his/her verbal consent in talking about cancer and its treatment. They were assured of the confidentiality of the information they provided. If plants were mentioned for their anticancer purposes, a botanical sample was collected. These specimens were pressed and preserved for later identification at the National Herbarium, Addis Ababa University, Addis Ababa, and a voucher specimen of each plant was deposited in the institute. All botanical names have been transcribed according to the nomenclature system used by the Plant List (http://www.theplantlist.org).
2.3. Data Analysis
The relative importance of medicinal plants used in the management of cancer-like symptoms in study areas was assessed using the relative frequency of citation (RFC), use value (UV), informants consensus factor (ICF), and cultural importance index (CI).
2.3.1. Relative Frequency of Citation (RFC)
The RFC was calculated by dividing the number of informants that cite a particular plant species (FC) by the total number of informants in the survey (N) [29]:
| (1) |
2.3.2. Use Value (UV)
The use value demonstrates the relative importance of plant species to treat particular ailment, and it is determined by the following formula [30]:
| (2) |
where “UV” stands for the use value of a species, “Ui” stands for the number of use reports cited by informants for that plant species, and “Ni” is the total number of informers who reported the particular plant species i.
2.3.3. Informant Consensus Factor (ICF)
Informant consensus factor (ICF) was calculated to determine the homogeneity of the information collected about particular plant species to treat specific ailment. It was estimated using the following formula [31]:
| (3) |
where Nur is the number of use reports of informants for particular ailment category and Nt refers to the number of species used for the ailment category by all informants.
2.3.4. Cultural Importance Index (CI)
Cultural importance index (CI) is calculated by the sum of the use reports (UR) of informants mentioning each species use (from i1 to iN) in each use category and adding all the UR of each category (from u1 to uNC) divided by the total number of informants N. This index is determined by the following formula [29]:
| (4) |
where CI is an ethnobotanical index that indicates the spread of the use along with the diversity of uses of each species.
3. Results
The informants consisted of 66 male and 8 female traditional healers and they were divided into three age groups: 20–40, 41–60, and ≥ 61 years. Out of 74 interviewed traditional healers, most of them (N%) were adults aged between 41 and 60 years. Majority of the respondents (70.2%) gained their knowledge from family members and 82% of all interviewed respondents practiced ethnomedicine for more than 25 years. More than 70% of the respondents were either only at their primary level of education or did not have a formal education at all (Figure 2). Traditional healers usually used their intuition and relied on the chronicity and growth of external mass, as a means to diagnose cancer. Lumpy growth was the most commonly cited criteria used to diagnose cancer, followed by ulcerative wounds and bleeding (Table 2). However, there were instances where some of the healers claimed to have treated patients already diagnosed with cancer at modern health institutions. Traditional healers identified cancer as “Nekersa” in Bahir Dar Zuria and Filiklik, “Naqarsa” in Bale Robe and Goba, “Sissac” in Gewane, “Xoka or Toka” in Doyo Gena, “Balamo” in Wondo Genet, “Kums or niamt” in North Bench, and “Kanser” in Sheko and Sodo Zuria district. Out of the 6 specific cancer types (skin, breast, lung, cervical, throat, and intestinal) claimed to be treated by the respondents, skin cancer was a dominant one followed by breast cancer.
Figure 2.
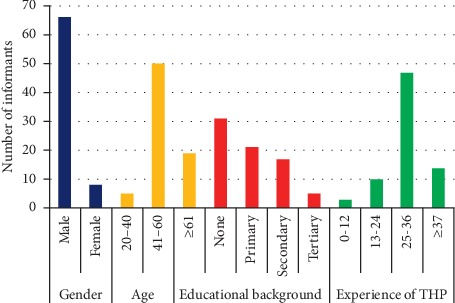
Demographic details of the interviewed informants.
Table 2.
Symptoms that are used by traditional healers to diagnose cancer.
| Cancer types | Reported symptoms | Number of traditional healers |
|---|---|---|
| Skin | Lumpy growth | 32 |
| Spreading pea-sized growth | 1 | |
| Ulcerative growth and oozing blood | 1 | |
|
| ||
| Breast | Lumpy growth | 17 |
| Lumpy growth on one breast and progressive weight loss | 1 | |
| Ulcerative wounds on breast | 5 | |
| Ulcerative wounds on breast and swelling on armpit and neck | 1 | |
| The patient was receiving anticancer treatment for breast cancer in hospital | 12 | |
|
| ||
| Cervical | Foul-smelling bloody vaginal discharge, pain during sexual intercourse, and weight loss | 1 |
|
| ||
| Colon | Chronic rectal bleeding and weight loss | 1 |
|
| ||
| Lung | Coughing up blood | 1 |
|
| ||
| Throat | Coughing and swelling on the neck | 1 |
A total of 53 plant species belonging to 30 families were reported for their anticancer use (Table 3). The result of this study showed that shrubs (49.1%), herbs (33.9%), trees (13.2%), and climbers (3.8%) were the main sources of potential anticancer medicinal plants. This study also indicated that leaves (56.7%) were the most commonly used plant parts followed by roots (21.7%), bark (6.7%), stem (1.7%), seeds (1.7%), whole plant (1.7%), leaves and roots (5%), leaves or stem (1.7%), and leaves or seeds (1.7%) (Figure 3). Most of the reported plants occurred naturally in wild (96.2%); however, cultivation was also a source (3.8%). Reported medicinal plants have been traditionally claimed to be used to treat different types of ailments including cancer. However, only few have been scientifically investigated for their antiproliferative or cytotoxic activity (Table 4). While comparing the amount and distribution of anticancer plants in the past ten years, regardless of the study areas, all respondents believed that the amount and distribution of these plants are reduced.
Table 3.
List of candidate medicinal plants traditionally used for cancer treatment in the study areas.
| Voucher number | Botanical name (family) | Vernacular name | Districts | Growth form | Habitat | Parts used | Preparation | Type of cancer treated | Application | UV | RFC | CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acanthaceae | ||||||||||||
|
| ||||||||||||
| Bele-047 | Justicia schimperiana (Hochst. ex Nees) T. Anderson | Kitkit | North Bench | Shrub | Wild | Roots | Fresh roots are crashed and boiled, and the cool decoction is drunk before meal | Lung | Oral | 1 | 0.027 | 0.067 |
| Bele-057 | Justicia schimperiana (Hochst. ex Nees) T. Anderson | Gulbana | Doyo Gena | Shrub | Wild | Leaves | Fresh leaves are pounded, and the juice is applied on the affected area | Skin | Topical | |||
|
| ||||||||||||
| Aloaceae | ||||||||||||
|
| ||||||||||||
| Bele-060 | Aloe sp. | Gurta waqota | Doyo Gena | Shrub | Wild | Leaves | Fresh roots are crashed, and the sap is applied on the affected area | Skin | Topical | 6 | 0.014 | 0.081 |
|
| ||||||||||||
| Amaranthaceae | ||||||||||||
|
| ||||||||||||
| Bele-044 | Achyranthes aspera L. | Koch ashite | Mizan Aman | Herb | Wild | Leaves | Leaves are roasted on metal plate, pounded into powder, mixed with animal butter, and smeared on the affected part | Skin | Topical | 4 | 0.014 | 0.054 |
|
| ||||||||||||
| Apiaceae | ||||||||||||
|
| ||||||||||||
| Bel-046 | Centella asiatica (L.) Urb. | Gorongoch | Sheko | Herb | Wild | Leaves | Young leaves are crashed, and the sap sniffed | Throat | Intranasal | 2 | 0.014 | 0.027 |
| Bel-002 | Hydrocotyle mannii Hook.f | Ye'ti medhanit | North Bench | Herb | Wild | Leaves | Young leaves are crashed and applied on the affected area | Skin | Topical | 1 | 0.014 | 0.014 |
|
| ||||||||||||
| Apocynaceae | ||||||||||||
|
| ||||||||||||
| Bel-003 | Acokanthera schimperi (A.DC.) Schweinf. | Merenz | Bahir Dar Zuria | Shrub | Wild | Leaves | Young leaves are crashed and applied | Skin | Topical | 0.5 | 0.027 | 0.027 |
| Bel-009 | Carissa spinarum L. | Agam | Bahir Dar Zuria | Shrub | Wild | Leaves | Leaves are crashed and infused in cold water overnight and drunk before meal and applied on the affected area | Skin | Oral | 1 | 0.027 | 0.027 |
|
| ||||||||||||
| Asclepiadaceae | ||||||||||||
|
| ||||||||||||
| Bel-040 | Calotropis procera (Aiton) Dryand. | Qumbo | Gewane | Shrub | Wild | Roots | Fresh roots are crashed, and the sap is applied on the affected area | Breast | Topical | 3 | 0.014 | 0.027 |
| Bel-036 | Pentarrhinum insipidum E. Mey. | Barohula | Gewane | Shrub | Wild | Roots | Fresh roots are crashed, and the sap is applied on the affected area | Breast and skin | Topical | 1 | 0.014 | 0.014 |
| Bel-037 | Echidnopsis dammanniana Sprenger | Mureli | Gewane | Herb | Wild | Stem | Stems are cut, and the sap is applied | Skin | Topical | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Asphodelaceae | ||||||||||||
|
| ||||||||||||
| Bel-020 | Kniphofia foliosa Hochst. | Shushube | Bale Goba | Shrub | Wild | Roots | Dry roots are pounded, and the powder is mixed with honey | Cervical and breast | Oral | 1 | 0.027 | 0.027 |
| Asteraceae | ||||||||||||
| Bel-045 | Acmella caulirhiza Delile | Kust asht | Mizan Aman | Shrub | Wild | Leaves | Young leaves are chewed by the healer and spit on | Breast | Topical | 0.67 | 0.04 | 0.054 |
| Bel-049 | Acmella caulirhiza Delile | Bitisa | Wondo Genet | Shrub | Wild | Leaves | Fresh leaves are crashed and infused in cold water | Breast | Oral | |||
| Bel-030 | Artemisia absinthium L. | Natrara | Sodo Zuria | Herb | Wild | Leaves | Dried leaves are ground and macerated in coffee or tea | Breast | Oral | 2 | 0.014 | 0.027 |
| Bel-029 | Artemisia afra Jacq. ex Willd. | Agufa | Doyo Gena | Herb | Wild | Leaves | Dried leaves are ground and macerated in coffee or tea | Breast | Oral | 1 | 0.014 | 0.014 |
| Bel-031 | Artemisia annua L. | Artemisia | Sodo Zuria | Tree | Cultivated | Leaves | Dried leaves will be ground and decocted in hot water | Breast | Oral | 1 | 0.014 | 0.014 |
| Bel-021 | Cineraria abyssinica Sch.Bip. ex A.Rich. | Unknown | Bale Robe | Herb | Wild | Leaves | Fresh leaves are pounded, and the sap is applied on the affected area | Skin | Topical | 1.5 | 0.027 | 0.054 |
| Bel-058 | Guizotia scabra (Vis.) Chiov. | Sheshota | Doyo Gena | Shrub | Wild | Leaves | Fresh leaves are pounded, and the sap is applied on the affected area | Skin | Topical | 1 | 0.014 | 0.014 |
| Bel-034 | Solanecio gigas (Vatke) C. Jeffrey | Arbaba | Doyo Gena | Shrub | Wild | Leaves | Fresh leaves are pounded and the sap is applied on the affected area | Skin | Topical | 2 | 0.014 | 0.027 |
| Bel-025 | Vernonia auriculifera Hiern | Barawa | Doyo Gena | Shrub | Wild | Leaves | Fresh leaves are pounded, and the sap is applied on the affected area | Skin | Topical | 1.33 | 0.041 | 0.081 |
| Bel-056 | Vernonia auriculifera Hiern | Reji | Wondo Genet | Shrub | Wild | Leaves | Fresh leaves are chewed by the healer and spit on | Skin | Topical | |||
|
| ||||||||||||
| Capparidaceae | ||||||||||||
|
| ||||||||||||
| Bel-039 | Cleome brachycarpa (Forssk.) Vahl ex DC. | Berbere | Gewane | Herb | Wild | Leaves | Fresh leaves are pounded, and the sap is applied on the affected area | Breast and skin | Topical | 3 | 0.014 | 0.014 |
|
| ||||||||||||
| Commelinaceae | ||||||||||||
|
| ||||||||||||
| Bel-026 | Commelina benghalensis L. | Laluncha | Doyo Gena | Herb | Cultivated | Roots | Fresh roots are pounded, and the sap is applied on the affected area | Skin | Topical | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Crassulaceae | ||||||||||||
|
| ||||||||||||
| Bel-019 | Kalanchoe petitiana A. Rich. | Anchura | Bale Goba | Shrub | Wild | Leaves | Fresh leaves are roasted for 2 minutes and applied on the affected area | Breast and skin | Topical | 1.5 | 0.027 | 0.041 |
|
| ||||||||||||
| Euphorbiaceae | ||||||||||||
|
| ||||||||||||
| Bel-012 | Croton macrostachyus Hochst. ex Delile | Bisana | Filiklik | Tree | Wild | Leaves or stem | Fresh leaves or succulent stems are crashed, and the sap is applied on the affected area | Breast and skin | Topical | 0.75 | 0.1 | 0.16 |
| Bel-035 | Croton macrostachyus Hochst. ex Delile | Besena | Doyo Gena | Tree | Wild | Bark | Dry bark is pounded, and the powder is applied on the affected area | Skin | Topical | |||
| Bel-048 | Croton macrostachyus Hochst. ex Delile | Masichoo | Wondo Genet | Tree | Wild | Leaves | Fresh leaves are crashed, macerated in cold water, and drunk | Breast and skin | Oral | |||
| Bel-032 | Euphorbia schimperiana Scheele | Gendalelata | Doyo Gena | Shrub | Wild | Roots | Fresh roots are pounded, and the sap is applied on the affected area | Skin | Topical | 1 | 0.014 | 0.014 |
|
| ||||||||||||
| Fabaceae | ||||||||||||
|
| ||||||||||||
| Bel-014 | Albizia schimperiana Oliv. | Sessa | Filiklik | Tree | Wild | Leaves | The mixture of fresh leaves of Albizia schimperiana and Carissa spinarum is macerated in cold water for 2 days, and the macerated liquid is drunk | Breast, intestinal, and skin | Oral | 4 | 0.014 | 0.014 |
| Bel-004 | Calpurnia aurea (Aiton) Benth. | Digita | Bahir Dar Zuria | Shrub | Wild | Leaves or seeds | Dry leaves or seeds are ground, macerated in cold water, and drunk | Breast | Oral | 2 | 0.014 | 0.027 |
| Bel-023 | Crotalaria agatiflora Schweinf. | Unknown | Bale Goba | Shrub | Wild | Seeds | Dry seeds are ground, mixed with honey, and applied | Skin | Topical | 1 | 0.014 | 0.014 |
| Bel-028 | Crotalaria incana L. | Chelke | Doyo Gena | Shrub | Wild | Leaves | Fresh leaves are crashed, and the sap is applied on the affected area | Skin | Topical | 1 | 0.014 | 0.014 |
| Bel-007 | Senna singueana (Delile) Lock | Gefa | Bahir Dar Zuria | Shrub | Wild | Leaves | Fresh leaves are crashed, macerated, and drunk | Skin | Oral | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Lamiaceae | ||||||||||||
|
| ||||||||||||
| Bel-043 | Ajuga leucantha Lukhoba | Tiks asht | North Bench | Herb | Wild | Leaves | Fresh leaves are crushed, and the sap is applied on the affected area | Breast | Topical | 1 | 0.014 | 0.014 |
| Bel-024 | Leonotis ocymifolia (Burm.f.) Iwarsson | Armagusa | Bale Goba | Herb | Wild | Leaves | Fresh leaves are crashed, macerated overnight, and drunk | Breast and skin | Oral | 3 | 0.014 | 0.014 |
| Bel-054 | Ocimum gratissimum L. | Mekedesisa | Wondo Genet | Herb | Wild | Roots | Fresh roots are crushed, boiled, and drunk | Skin | Oral | 2 | 0.014 | 0.027 |
| Bel-059 | Pycnostachys abyssinica Fresen. | Tontona | Doyo Gena | Herb | Wild | Leaves | Fresh leaves are crushed, and the sap is applied on the affected area | Skin | Topical | 2 | 0.014 | 0.027 |
| Bel-042 | Salvia nilotica Juss. ex Jacq. | Barnbanch | North Bench | Shrub | Wild | Whole plant | Dry plant parts are ground, mixed with honey, and applied | Breast | Topical | 2 | 0.014 | 0.027 |
| Bel-022 | Thymus schimperi Ronniger | Tosigne | Bale Goba | Herb | Wild | Leaves | Dry leaves are decocted and drunk | Breast | Oral | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Malvaceae | ||||||||||||
|
| ||||||||||||
| Bel-051 | Sida schimperiana Hochst. ex A. Rich. | Kotijebessa | Wondo Genet | Shrub | Wild | Roots and leaves | Fresh leaves and roots are crashed, macerated, and drunk | Breast and skin | Oral | 4 | 0.014 | 0.027 |
|
| ||||||||||||
| Melianthaceae | ||||||||||||
|
| ||||||||||||
| Bel-001 | Bersama abyssinica Fresen. | Azamir | Bahir Dar Zuria | Shrub | Wild | Bark | Dry bark is ground, macerated, and drunk before meal | Breast | Oral | 1 | 0.014 | 0.014 |
|
| ||||||||||||
| Moraceae | ||||||||||||
|
| ||||||||||||
| Bel-008 | Dorstenia barnimiana Schweinf. | Work Bemeda | Bahir Dar Zuria | Herb | Wild | Roots | Dry roots are ground, mixed with water and honey, and drunk, or dry roots are ground, mixed with honey, and applied on the affected area | Breast | Oral or topical | 0.6 | 0.068 | 0.12 |
|
| ||||||||||||
| Myrtaceae | ||||||||||||
|
| ||||||||||||
| Bel-006 | Syzygium guineense (Willd.) DC. | Dokima | Bahir Dar Zuria | Tree | Wild | Leaves and roots | Dry leaves and roots of Syzygium guineense and dry leaves of Osyris quadripartita are ground, mixed, decocted, and drunk | Skin | Oral | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Oxalidaceae | ||||||||||||
|
| ||||||||||||
| Bel-052 | Oxalis corniculata L. | Qinta | Wondo Genet | Herb | Wild | Leaves and roots | Fresh leaves and roots are crashed and applied with a bandage | Breast | Topical | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Polygonaceae | ||||||||||||
|
| ||||||||||||
| Bel-018 | Rumex nervosus Vahl | Emboacho | Filiklik | Shrub | Wild | Roots | Dry roots are ground, macerated, and drunk | Skin | Oral | 3 | 0.014 | 0.041 |
| Bel-033 | Rumex nepalensis Spreng. | Goecho | Doyo Gena | Herb | Wild | Roots | Dry roots are ground and taken with food | Colon | Oral | 1.5 | 0.027 | 0.041 |
| Bel-053 | Rumex nepalensis Spreng. | Sharibicho | Wondo Genet | Herb | Wild | Bark | Fresh bark is crashed and squeezed, and the sap is applied | Skin | Topical | |||
|
| ||||||||||||
| Ranunculaceae | ||||||||||||
|
| ||||||||||||
| Bel-010 | Clematis simensis Fresen. | Yeazo Hareg | Bahir Dar Zuria | Climber | Wild | Leaves | Fresh roots of Dorstenia barnimiana mixed with fresh leaves of Clematis simensis, pounded, and applied | Breast | Topical | 0.67 | 0.041 | 0.054 |
|
| ||||||||||||
| Rosaceae | ||||||||||||
|
| ||||||||||||
| Bel-011 | Prunus africana (Hook.f.) Kalkman | Tikur enchet | Bahir Dar Zuria | Tree | Wild | Bark | Dry bark is ground, decocted, and drunk | Breast and skin | Oral | 3 | 0.014 | 0.014 |
| Rutaceae | ||||||||||||
| Bel-016 | Clausena anisata (Willd.) Hook.f. ex Benth. | Limich | Filiklik | Shrub | Wild | Leaves | Dry leaves are ground, mixed with honey, and eaten | Breast | Oral | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Santalaceae | ||||||||||||
|
| ||||||||||||
| Bel-013 | Osyris quadripartita Salzm. ex Decne. | Keret | Filiklik | Shrub | Wild | Leaves | Dry leaves are ground, decocted, and drunk | Breast | Oral | 2 | 0.027 | 0.027 |
|
| ||||||||||||
| Sapindaceae | ||||||||||||
|
| ||||||||||||
| Bel-005 | Dodonaea viscosa subsp. angustifolia (L.f.) J.G.West | Kitkita | Bahir Dar Zuria | Tree | Wild | Roots | Dry roots are ground, mixed with honey, and applied or dry roots are ground, decocted, and drunk | Breast, skin and cervical | Topical or oral | 1 | 0.014 | 0.041 |
|
| ||||||||||||
| Simaroubaceae | ||||||||||||
|
| ||||||||||||
| Bel-017 | Brucea antidysenterica J.F.Mill. | Abalo | Filiklik | Tree | Wild | Leaves | Dry leaves are ground, pasted with cold water, and applied | Skin | Topical | 4 | 0.014 | 0.054 |
|
| ||||||||||||
| Solanaceae | ||||||||||||
|
| ||||||||||||
| Bel-027 | Discopodium penninervium Hochst. | Chechanga | Doyo Gena | Shrub | Wild | Leaves | Fresh leaves are crashed and applied | Skin | Topical | 1 | 0.014 | 0.014 |
|
| ||||||||||||
| Thymelaeaceae | ||||||||||||
|
| ||||||||||||
| Bel-055 | Gnidia involucrata Steud. ex A.Rich. | Bito | Bahir Dar Zuria | Herb | Wild | Roots | Dry roots are ground, mixed with honey, and eaten | Breast | Oral | 0.5 | 0.027 | 0.027 |
|
| ||||||||||||
| Verbenaceae | ||||||||||||
|
| ||||||||||||
| Bel-050 | Lantana trifolia L. | Hanshebello | Wondo Genet | Shrub | Wild | Leaves | Fresh leaves are ground, macerated in cold spring water, and drunk | Breast and skin | Oral | 2 | 0.014 | 0.014 |
| Bel-015 | Lippia adoensis Hochst. | Kessie | Filiklik | Shrub | Wild | Leaves | Dry leaves are ground, macerated in cold water, and drunk | Skin | Oral | 2 | 0.014 | 0.027 |
|
| ||||||||||||
| Vitaceae | ||||||||||||
|
| ||||||||||||
| Bel-038 | Cyphostemma serpens (Hochst. ex A.Rich.) Desc. | Eiriti | Gewane | Climber | Wild | Roots | Dry roots are ground, pasted with honey and eaten, and applied | Skin | Oral and topical | 1 | 0.014 | 0.014 |
UV = use value; RFC = relative frequency of citation; CI = cultural importance index.
Figure 3.
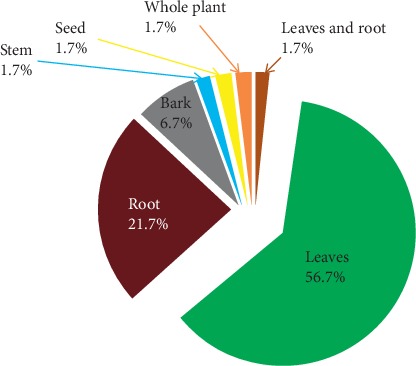
Frequency of plant parts used for the preparation of medicinal remedy.
Table 4.
Cross-reference of cancer treatment candidate plant species collected from the study areas with the published literature.
| Botanical name (family) | Biological activity/chemical constituents | Illnesses/symptoms claimed to be treated traditionally |
|---|---|---|
| Justicia schimperiana (Hochst. ex Nees) T. Anderson (Acanthaceae) | Saponins, alkaloids, terpenoids and flavonoids [32] In vitro cytotoxicity [33]; in vitro antioxidant activity on DPPH assay [34]; in vivo suppression of parasitaemia on Plasmodium berghei-infected mice in the 4-day suppressive test [32]; and in vivo hepatoprotective activity in mice intoxicated with CCL4 [35] |
Wound [15, 21]; rabies [15, 18–20, 36–39]; jaundice [15, 16, 21, 23, 38, 40]; gonorrhea [17, 36, 39]; liver cirrhosis [18, 26]; seizure [19, 41]; stomach ache [15, 25, 38]; helminths [15, 42, 43]; skin burn/lesion [23, 44]; arthritis [21, 23]; hepatitis [45, 46]; evil eye [15, 46]; dysentery [15, 21]; malaria [36, 39]; common cold, asthma, and headache [36, 39, 47]; otitis [48]; toothache [49]; and rheumatism [50] |
|
| ||
| Aloe sp. (Aloaceae) | Anthrones and chromones [51], pyrones, coumarins, alkaloids, glycoproteins, naphthalenes, and flavonoids [52] 7‐O‐methylaloeresin showed in vitro antioxidant activity in DPPH assay [51], and methanol and ethanol extract showed in vivo parasitaemia suppression on Plasmodium berghei-infected mice in the 4-day suppressive test [53, 54] |
Wound [21, 55]; eye disease [21, 46, 48, 56]; snake bite [21, 48, 56]; malaria [20, 21, 44, 48, 54]; easing labour [44]; tropical ulcer, colon cleaner, and gallstone [48]; amoeba, abdominal pain, impotence, and urine retention [21]; dandruff [46, 56], hemorrhoids and hepatitis B [46]; ascariasis [21]; diabetes [54]; asthma [55]; foot strain [57, 58]; wart and anthrax [20]; external injury [59]; and liver swelling, splenomegaly, and skin inflammation [56] |
|
| ||
| Achyranthes aspera L. (Amaranthaceae) | Phytosteroids, polyphenols, and saponins [60] Methanol extracts have showed in vivo wound healing activity [61] |
Bleeding [21, 24, 26, 62–64]; retained placenta [21, 62]; stomach ache and external swelling [17]; rhesus factor incompatibility in pregnancy [40, 55]; epistaxis [19]; hepatitis and evil eye [24]; tonsillitis [21, 57]; snake bite and paralysis [21]; dysentery [59]; herpes zoster [26]; anthrax [21, 49]; nasal infection and ophthalmic infection [64]; excessive menstruation and tape worm infection [15]; and gonorrhea [65] |
|
| ||
| Centella asiatica (L.) Urb. (Apiaceae) | Terpenoids (triterpenes, asiaticoside, centelloside, madecassoside, brahmoside, brahminoside (saponin glycosides), asiaticentoic acid, centellic acid, centoic acid, madecassic acid, terminolic acid, betulic acid, β-caryophyllene, trans-β-farnesene and germacrene D (sesquiterpenes), α-pinene, and β-pinene [66, 67] Methanol extract inhibited the proliferation of human gastric adenocarcinoma (MK-1), human uterine carcinoma (HeLa), and murine melanoma (B16F10) cells in vitro [68]; aqueous extracts induced apoptosis in colonic crypts and exerted chemopreventive effect on colon tumorigenesis in male F344 rats [69] |
Genital infection and lymphadenitis [63]; topical swelling [26, 70]; gastritis, headache, and evil eye [70]; bleeding [40]; wound [24]; abdominal ache [71]; meningitis [72]; and tinea corporis [47] |
|
| ||
| Hydrocotyle mannii Hook.f (Apiaceae) | No previous reports | Eye infection [63] and cataract [72] |
| Acokanthera schimperi (A.DC.) Schweinf. (Apocynaceae) | In vitro cytotoxicity [73]; in vitro antiviral activity against coxsackie B3, influenza A, and herpes simplex type1 virus [74]; in vitro antimicrobial activity against Staphylococcus aureus, Pseudomonas aeruginosa, Trichophyton mentagrophytes [75]; and in vivo parasitaemia suppression in Plasmodium berghei-infected mice [76] | Wound [16, 44, 77, 78]; hepatitis [15, 16, 22, 44]; gonorrhea [19, 25]; evil eye [62]; bone fracture [24]; hemorrhoids [44]; scabies [21]; malaria and tonsillitis [48, 56]; psychiatric disease [55]; and skin diseases [65] |
|
| ||
| Carissa spinarum L. (Apocynaceae) | In vitro antioxidant activity on DPPH assay and in antiproliferative activity [79] | Throat cancer [23, 80]; evil eye [16, 21, 24, 49, 62, 70, 72, 81]; snake bite [23, 80]; gonorrhea [20, 65]; stomach ache [20, 70]; impotence and headache [20]; tonsillitis [17, 56, 70]; wound and febrile illness [16]; bleeding after delivery [44]; muscle cramps [49]; toothache [47]; and premature ejaculation [56] |
|
| ||
| Calotropis procera (Aiton) Dryand. (Asclepiadaceae) | Latex contains phytochemicals such as alkaloids, sterols, fatty acids, starches, sugars, oils, tannins, resins, and gums, and enzymatic proteins such as proteases, chitenases, lipases, peptidases, esterase, peroxidases, papain, hevein, and lectins [82] In vivo hepatoprotective [83]; hypoglycemic effect [84]; strong anti-implantation (antifertility) [85]; crude latex showed antioxidant and antiapoptotic activities against the toxicity of 4-nonylphenol [86] |
Wound [16, 21, 81]; hemorrhoids [16, 19, 44]; wart [16, 57]; snake bite [23, 87]; kidney stone, tuberculosis, and scabies [16]; swelling [58]; skin rash [21, 49]; tinea capitis [21] |
|
| ||
| Pentarrhinum insipidum E. Mey. (Asclepiadaceae) | No previous reports | |
|
| ||
| Echidnopsis dammanniana Sprenger (Asclepiadaceae) | No previous reports | Snake bite [56] |
|
| ||
| Kniphofia foliosa Hochst. (Asphodelaceae) | 2-Acetyl-1-hydroxy-8-methoxy-3-methylnaphthalene, 10-(chrysophanol-7′-yl)-10-(ξ)-hydroxychrysophanol-9-anthrone, chryslandicin, knipholone, and chrysophanol [88] 10-(Chrysophanol-7′-yl)-10-(ξ)- hydroxychrysophanol-9-anthrone showed in vitro antiplasmodial activity against chloroquine-sensitive 3D7 strain of Plasmodium falciparum and knipholone selectively inhibited leukotriene metabolism in in vitro a human blood assay [88]; knipholone anthrone showed in vitro cytotoxicity [89] and antioxidant activity on DPPH assay [90] |
No previous reports |
|
| ||
| Acmella caulirhiza Delile (Asteraceae) | Unsaturated alkylamides like spilanthol and N-isobutylnona-2E,4E-dien-8ynamide [91] In vitro antiplasmodial activity [92] |
Swelling [15]; tonsillitis [20, 63]; and toothache [40, 87] |
|
| ||
| Artemisia absinthium L. (Asteraceae) | Camphor, davanone, ethyl (E)-cinnamate, (E)-nerolidol, and chamazulene [93] Essential oils showed in vitro antiparasitic effects against promastigote and axenic amastigote forms of Leishmania donovani and Leishmania aethiopica and in vitro cytotoxicity on THP-1 (human leukaemia) cell lines [93]; and in vitro cytotoxicity on human leukaemia cell lines [94] |
Hypertension, stomach ache, severe abdominal cramp [18] and sour throat [40] |
|
| ||
| Artemisia afra Jacq. ex Willd. (Asteraceae) | Epoxylinalol and dihydrocostunolide [94]; camphor, davanone, bornyl acetate, 4-terpineol, and chamazulene [95] In vitro cytotoxicity on human leukaemia cell lines [73]; and in vitro antioxidant effect on DPPH assay [95] |
Stomach ache [18, 42]; evil eye [16, 17, 62]; headache [42, 77]; eye disease, tinea capitis infection, hematuria, and stabbing pain [77]; antifertility agent [33]; malaria [42, 62]; ascariasis [18]; epilepsy and febrile illness [46, 65] |
| Artemisia annua L. (Asteraceae) | In vitro inhibition of immune mediators of angiogenesis [96]; the sesquiterpene (Z)-7-acetoxy-methyl-11-methyl-3-methylene-dodeca-1,6,10-triene showed moderate cytotoxic activities against the human tumor cell lines of HO8910 (ovary), 95-D (lung), QGY (liver), and HeLa (cervix) by MTT assay and induced apoptosis on 95-D tumor cells [97]; artemisinin and quercetagetin 6,7,3′,4′-tetramethyl ether showed significant cytotoxicity against P-388, A-549, HT-29, MCF-7, and KB tumor cells [98] | No previous reports |
|
| ||
| Cineraria abyssinica Sch.Bip. ex A.Rich. (Asteraceae) | In vitro radical scavenging activity on DPPH assay [99]; flavonoidal glycoside (rutin) showed in vitro antibacterial activity [100] | No previous reports |
|
| ||
| Guizotia scabra (Vis.) Chiov. (Asteraceae) | In vitro cytotoxicity on human leukaemia cell lines [73], and in vitro antiviral activity [101] | Wound [20]; epilepsy [40]; and ectoparasite infestation [47] |
|
| ||
| Solanecio gigas (Vatke) C. Jeffrey (Asteraceae) | In vitro antiviral activity against human immunodeficiency virus type 1 and type 2 cytotoxicity on human T-lymphocytic MT-4 cell lines [102] | Skin diseases [62]; retained placenta [40]; hepatitis [64]; evil eye [15] |
|
| ||
| Vernonia auriculifera Hiern (Asteraceae) | Tannins, flavonoids, terpenoids, and saponins [103] | Toothache [72]; snake bite [42]; skin cut [47] |
|
| ||
| Cleome brachycarpa (Forssk.) Vahl ex DC. (Capparidaceae) | No previous reports | |
|
| ||
| Commelina benghalensis L. (Commelinaceae) | Phlobatannins, carbohydrates, tannins, glycosides, volatile oils, resins, balsams, flavonoids, and saponins [104] Ethanol extract showed in vivo sedative and anxiolytic activity [105] |
Helminths [65]; skin infection [72] |
|
| ||
| Kalanchoe petitiana A. Rich. (Crassulaceae) | Polyphenols, alkaloids, flavonoids, tannins, saponins, and steroids [106] In vitro antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, and Staphylococcus aureus [75]; and in vivo wound healing activity [106] |
Breast and skin cancer [107]; swelling [40, 77]; tapeworm infection, trachoma, and syphilis [77]; lymphadenopathy and evil eye [22]; sore muscles [108]; itching skin [63]; and bone fracture [23] |
|
| ||
| Croton macrostachyus Hochst. ex Delile (Euphorbiaceae) | Ethanol extract showed in vitro antioxidant activity on DPPH assay [79] | Tumor, rabies, and wart [24]; skin cancer and wound [17]; gonorrhea [20, 23, 62]; headache [18, 109]; snake bite [18, 72]; malaria [16, 18–20, 110]; helminths [18, 111]; tinea nigra [40]; ringworm [17, 62]; tinea versicolor [16, 25]; heart failure [62]; bleeding [18, 24]; hepatitis [16, 18, 24]; stomach ache [16, 18, 23]; diarrhea [16, 18]; lymph adenitis and rheumatism [18]; bloat, scabies, and urine retention [16]; retained placenta and leprosy [19] |
|
| ||
| Euphorbia schimperiana Scheele (Euphorbiaceae) | In vitro cytotoxic effect against breast cancer (MCF7), hepatocellular carcinoma (HEPG2), and cervix cancer (HELA) cells [112] | Syphilis [108] |
|
| ||
| Albizia schimperiana Oliv. (Fabaceae) | In vitro cytotoxicity on human leukaemia cells [73] | Evil eye [20]; kidney infection and liver cirrhosis [18] |
| Calpurnia aurea (Aiton) Benth. (Fabaceae) | 3β,4α,13α-Trihydroxylupanine, calpaurine, lupinine, and epilupinine calpurmenine and calpurmenine pyrrolecarboxylic acid ester, 13-hydroxylupanine, its tiglate and pyrrolecarboxylic acid esters (calpumine), virgiline and virgiline pyrrolecarboxylic acid ester [113]; 4β-hydroxy-13α-O-(2′-pyrrolylcarbonyl)-lupanine (digittine) and 4β,13α-dihydroxylupanine [114]; alkaloids, tannins, flavonoids, and saponins [35] Methanol extract showed in vitro antimicrobial activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa [75] and type 1 and type 2 human immunodeficiency virus and showed cytotoxicity on human T-lymphocytic MT-4 cell lines [102]; methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cells [73]; and ethanol extracts showed in vitro antioxidant activity on DPPH assay [79] |
Tumor [22, 26, 80]; stomach ache [21, 62, 70, 81]; wound and skin infection [62]; Gonorrhoea and syphilis [16], amoebiasis [16, 80]; ascariasis and gastric ulcer [23]; diarrhea [21, 38, 70]; scabies and pubic hair louse [40]; diabetes mellitus and hypertension [19]; herpes zoster, hemorrhoids and tinea capitis [21]; and swelling and tuberculosis [58] |
|
| ||
| Crotalaria agatiflora Schweinf. (Fabaceae) | Methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cells [73] | No previous reports |
| Crotalaria incana L. (Fabaceae) | Dihydrosenecionine isomer, nemorensine isomer, integerrimine and anacrotine [115] Methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cell lines [73] |
|
|
| ||
| Senna singueana (Delile) Lock (Fabaceae) | Methanol extracts showed in vitro antioxidant activity on DPPH assay [116] | Stomach ache [58, 62, 70]; wound and swellings [62]; teeth infection and sprain [58] |
|
| ||
| Ajuga leucantha Lukhoba (Lamiaceae) | No previous reports | Diarrhea [70] |
|
| ||
| Leonotis ocymifolia (Burm.f.) Iwarsson (Lamiaceae) | Methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cells [73] | Ascariasis [62], febrile illness [16, 62]; eye disease [16]; headache and neck ulcer [55]; and snake bite [15] |
|
| ||
| Ocimum gratissimum L. (Lamiaceae) | Essential oil contains constitutes γ-terpinene, β-phellandrene, limonene, and thymol and showed in vivo antiplasmodial activity against Plasmodium berghei infection [117] | Allergy reaction [18, 20]; rheumatism, headache and eye disease [18]; febrile illness and general malaise [40]; sun stroke [24]; malaria [44] |
|
| ||
| Pycnostachys abyssinica Fresen. (Lamiaceae) | No previous reports | Eye disease [18, 47]; ascariasis and wound [18]; diarrhea, stomach ache, amoebiasis, stomach bloating, and food poisoning [70]; headache [63] |
|
| ||
| Salvia nilotica Juss. ex Jacq. (Lamiaceae) | Essential oil contains germacrene D, guaiol, and trans-caryophyllene as major constituents and showed activity against both Gram-positive and Gram-negative pathogenic bacteria; the oil also showed in vitro antioxidant activity on DPPH assay [118] | Tonsillitis and constipation [62]; herpes simplex [18, 38]; wound [40]; lymphadenitis [63]; and hemorrhoids and diarrhea [65] |
|
| ||
| Thymus schimperi Ronniger (Lamiaceae) | Phenol and flavonoid compounds, and aqueous methanol extract showed in vitro radical scavenging ability, iron reducing power, and total antioxidant capacity [119] | Diabetes [62]; hypertension [18, 40]; tonsillitis [18]; toothache [18, 21]; abdominal pain [21]; and cough [38, 55] |
|
| ||
| Sida schimperiana Hochst. ex A. Rich. (Malvaceae) | No previous reports | “Shotelaye” (hydrops fetalis) [21, 22]; cough and fever [62]; diarrhea [18]; wound [25, 62]; bleeding and evil eye [24]; glandular disease and rabies [40]; amoebic dysentery, and liver disease [65]; paralysis [21]; epilepsy [43] |
| Bersama abyssinica Fresen. (Melianthaceae) | Flavonol glycosides isoquercetrin, hyperoside, quercetin-3-O-arabinopyranoside, kaempferol-3-O-arabinopyranoside, xanthone glycoside, mangiferin [115] Ethanol water extracts showed in vitro antioxidant activity on DPPH assay and antiproliferative activity on human liver carcinoma cell line and normal human fetal lung cells [79]; methanol extract showed in vitro antioxidant activity on DPPH assay [115], and antiviral activity against type 1 human immunodeficiency virus [102] |
Tumor, dysentery and roundworms [107, 109]; ascariasis [15, 38, 81, 109]; wound [20]; stomach ache [17]; snake bite and liver diseases [70]; tonsillitis [72]; bronchitis and febrile illness [42, 43] |
|
| ||
| Dorstenia barnimiana Schweinf. (Moraceae) | Phytochemical screening showed the presence of coumarins [34] | Cancer [26]; hepatitis, syphilis and rabies [25, 26]; skin cancer, dysentery, wart and fever [25]; pulmonary tuberculosis, leprosy, and stomach illness [22] |
|
| ||
| Syzygium guineense (Willd.) DC. (Myrtaceae) | Methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cells [73] and antimicrobial activity [120] | Stomach ache [17–19, 23]; diarrhea [15, 18, 19, 24], kidney infection, liver cirrhosis, and tonsillitis [18]; syphilis [23, 80]; malaria, hemorrhoid, internal worms, snake bite, and gonorrhea [65] |
|
| ||
| Oxalis corniculata L. (Oxalidaceae) | In vivo antitumor activity against Ehrlich ascites carcinoma on mice [121] | Wound [17]; arthritis [63]; tape worm infection [21] |
|
| ||
| Rumex nervosus Vahl (Polygonaceae) | Alkaloids, flavonoids, terpenoids, tannins, glycosides, and volatile oils [122] | Breast cancer, gastritis, and snake bite [16]; wart [15, 22]; hepatitis [49, 55]; skin rash [16, 21]; bleeding [15, 40, 81, 109]; wound [40, 49, 55, 62, 109, 110]; scabies and acne vulgaris [62]; ascariasis and herpes simplex [21]; stomach ache and dysentery [22]; diarrhea [49]; eye problems and round worm [55] |
|
| ||
| Rumex nepalensis Spreng. (Polygonaceae) | Anthraquinones, naphthalenes, tannins, stilbenoids [123] Ethanol water extracts showed in vitro antiproliferative activity on human liver carcinoma cell line and on normal human fetal lung cells and antioxidant activity on DPPH assay [79], and methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cells [73] |
Wound, ascariasis, abdominal bleeding, gastric ulcer, and hemorrhage [23, 80]; gastritis [18]; stomach problems [108]; leishmaniasis [25]; abdominal cramp and ear infection [63]; tonsillitis [18, 25] |
|
| ||
| Clematis simensis Fresen. (Ranunculaceae) | Triterpenoids, saponins, alkaloids, polyphenols, and unsaturated sterols [120] In vivo anti-inflammatory and antinociceptive activities [124] |
Cancer and hemorrhoid [15]; wart and evil eye [24, 40]; wound [15, 24, 40, 63, 81]; tonsillitis [62]; eye infection [63]; leg swelling, malaria, and mental illness [49]; stomach ache [47] |
|
| ||
| Prunus africana (Hook.f.) Kalkman (Rosaceae) | No previous reports | Benign prostatic hyperplasia and prostate gland hypertrophy [20]; cancer, respiratory disorders, bad breathe, diarrhea, gonorrhea, tuberculosis, and ear problems [22]; swelling [40]; wounds [19, 22]; tonsillitis [23, 80] |
|
| ||
| Clausena anisata (Willd.) Hook.f. ex Benth. (Rutaceae) | Carbazole alkaloids, peptide derivatives, sitosterol, and stigmasterol [125] Methanol and dichloromethane crude extracts showed in vitro cytotoxicity on human leukaemia cells [73] |
Skin irritation [20]; toothache [40]; ascariasis [19]; evil eye [24, 25, 63] |
|
| ||
| Osyris quadripartita Salzm. ex Decne. (Santalaceae) | Alkaloids, phenols, terpenoids, tannins, saponins, and flavonoids [126] Methanol extracts showed in vitro antimicrobial activity against Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Candida albicans, and Trichophyton mentagrophytes [11]; in vitro inhibition of NO production and cytotoxicity against MCF-7 and NCI-H460 cell lines [127] |
Cancer [62]; anaphylactic shock, evil eye, and epilepsy [18]; eczema [40]; toothache [46] |
| Dodonaea viscosa subsp. angustifolia (L.f.) J.G.West (Sapindaceae) | Alkaloids, terpenoids, saponins, tannins, sugars, phenolics, and flavonoids [128] Methanol extracts showed in vivo nonsensitizer effect in mice using the mouse ear swelling test method [129], in vitro antiviral effect against type 1 human immunodeficiency virus [102], and in vitro free radical scavenging activity on DPPH assay [128] |
Malaria [57] |
|
| ||
| Brucea antidysenterica J.F.Mill. (Simaroubaceae) | Flavonoids, amino acids, and vitamin C [130] In vitro antiplasmodial activity against Plasmodium berghei infection [131] |
Cancer/tumor [107]; wart [24]; rabies [18, 62]; leprosy [62] |
|
| ||
| Discopodium penninervium Hochst. (Solanaceae) | 5α,17β-Dihydroxy-6α,7α-epoxy-1-oxowitha-2,24-dienolide, withanone, and withanolide A [132], 5,6-epoxy-16-oxygenated withanolides, jaborosalactone-L, and 17-epiacnistin-A [133, 134]; 6α,7α-epoxy-1-oxo-5α,12α,17α-trihydroxywitha-2,24-dienolide and a coloratane sesquiterpene, 7α,11α-dihydroxy-4(13),8-coloratadien-12,11-olide, withanone, 5α,17β-dihydroxy-6α,7α-epoxy-1-oxowitha-2,24-dienolide, 7α,11α-dihydroxy-8-drimen-12,11-olide, withasomnine, and (E,Z)-9-hydroxyoctadeca-10,12-dienoic acid [135] Jaborosalactone-L showed cytotoxicity only to the murine macrophage cell line, RAW 264.7, but the 16α-oxygenated withanolides exhibited cytotoxicity to both human (COR-L23 and ECV 304) and murine (L929 and RAW 264.7) carcinoma cell lines with IC50 values ranging from 1.2 to 150 μM [136]. 6α,7α-Epoxy-1-oxo-5α,12α,17α-trihydroxy-witha-2,24-dienolide inhibited COX-2 and LTB4 formation; 7α,11α-dihydroxy-4(13),8-coloratadien-12,11-olide and withasomnine inhibited LTB4 biosynthesis but showed minor inhibition of COX-1 and COX-2 [135] |
Skin detoxification [62]; and liver disease [70] |
|
| ||
| Gnidia involucrata Steud. ex A.Rich. (Thymelaeaceae) | Flavonoids and glycosides [137] | Ascariasis, evil eye, anthrax, intestinal helminths, and gland swelling [18] |
|
| ||
| Lantana trifolia L. (Verbenaceae) | Flavone glycosides (scutellarein-7-O-β-D-apiofuranoside and apigenin-7-O-β-D-apiofuranosyl-(1⟶2)-β-D-apiofuranoside) and the flavone celtidifoline (5,6,40,50-tetrahydroxy-7,30-dimethoxyflavone) [138, 139] | Headache [70]; malaria [71] |
|
| ||
| Lippia adoensis Hochst. (Verbenaceae) | Limonene, perillaldehyde, piperitenone, and 2-methyl-6-methylene-2,7-octadien-4-one [140], sesquiterpene hydrocarbon (germacrene D) [141] Methanol extract showed in vitro cytotoxicity on human leukaemia cell lines [73], and antimicrobial activity against Staphylococcus aureus, Escherichia coli, and Pseudomonas aeruginosa [75]; water extracts showed in vivo protection/relieve against acetic acid induced writhing in mice model [142] |
Eczema, fungal infections, common cold, and cough [62]; intestine swelling [18]; gastrointestinal disorder [40]; abdominal irritation and acute stomach illness [46] |
|
| ||
| Cyphostemma serpens (Hochst. ex A.Rich.) Desc. (Vitaceae) | No previous reports | |
In the current study, the highest UVs were recorded for Aloe spp. (6), Albizia schimperiana (4), Sida schimperiana (4), Achyranthes aspera (4), Brucea antidysenterica (4), Cleome brachycarpa (3), Leonotis ocymifolia (3), and Prunus africana (3). The lowest UVs were obtained for Acokanthera schimperi, Acmella caulirhiza, Cineraria abyssinica, and Gnidia involucrata (Table 3). A total of 228 use reports have been documented and categorized into seven categories (Table 5). Among these, other ailments (46.3%) and skin cancer (26.5%) had the highest use reports. Furthermore, ICF values were also calculated and ranged from 0 to 0.42. The highest ICF values were recorded in other ailments (0.42) and breast cancer (0.32) followed by skin cancer (0.23) category (Table 5). The other ailments category comprises of diseases such as stomach ache, malaria, wart, swelling, wounds, evil eye, toothache, bleeding, gastrointestinal disorder, headache, bone fracture, cough, snake bite, herpes simplex, tonsillitis, hypertension, dandruff, fever, and hemorrhoid. The ICF value of the remaining four categories (lung cancer, colon cancer, cervical cancer, and throat cancer) was zero. Quantitative ethnobotanical indexes such as RFC and CI were calculated in this study to analyze the ethnobotanical information. According to RFC values, Croton macrostachyus (0.1), Vernonia auriculifera (0.04), Clematis simensis (0.04), and Acmella caulirhiza (0.04) are the most frequently cited among all reported plants. Croton macrostachyus (0.16), Dorstenia barnimiana (0.12), and Aloe spp. (0.08) rank 1st, 2nd, and 3rd in position, respectively, according to the CI reference. Our result also shows that the Pearson correlation coefficient of RFC was positively and negatively correlated to CI and UV, respectively (Table 6).
Table 5.
Informants consensus factor for different ailment categories.
| No. | Category | No. of species | % of all species | No. of use reports | % of all use reports | ICF |
|---|---|---|---|---|---|---|
| 1 | Skin | 25 | 30.5 | 32 | 26.5 | 0.23 |
| 2 | Breast | 20 | 24.4 | 29 | 23.9 | 0.32 |
| 3 | Cervical | 1 | 1.22 | 1 | 0.83 | 0 |
| 4 | Colon | 1 | 1.22 | 1 | 0.83 | 0 |
| 5 | Lung | 1 | 1.22 | 1 | 0.83 | 0 |
| 6 | Throat | 1 | 1.22 | 1 | 0.83 | 0 |
| 7 | Other disease | 33 | 40.2 | 56 | 46.3 | 0.42 |
| Total | 82 ∗ | 121 |
∗Each plant species may be listed in several categories.
Table 6.
Summary of stats for relative frequency of citation (RFC) and cultural importance index (CI).
| Mean | Standard deviation | Minimum | Maximum | |
|
| ||||
| UV | 1.8 | 1.1 | 0.5 | 6 |
| RFC | 0.02 | 0.015 | 0.014 | 0.1 |
| CI | 0.034 | 0.027 | 0.014 | 0.16 |
|
| ||||
| Association between RFC and CI by using Pearson correlation method | ||||
| UV | RFC | CI | ||
|
| ||||
| UV | 1 | |||
| RFC | −0.36∗ | 1 | ||
| CI | 0.003 | 0.858∗∗ | 1 | |
∗Correlation is significant at 0.05 level. ∗∗Correlation is significant at 0.001 level.
Most of the reported remedies, prepared from these plants, were either applied topically (50%) or taken orally (41.7%). The remaining remedies were prepared to be administered either topically or orally (3.3%), both topically and orally (1.7%), and intranasally (1.7%). Usually, fresh plants were finely chopped, dried, and pounded to powder form. Then, the powder of either one or the combination of more than one plant was either mixed with drinking water or pasted and applied topically. In other cases, fresh plant parts were decocted and taken orally or crushed and applied topically. Water was the main medium in preparation of most remedies and additives like honey, milk, and butter were also used. To determine the amount of plant parts used to prepare remedies, traditional healers used spoon, fingertip, and number (in case of fresh leaves). Adverse effects reported by respondents include vomiting, diarrhea, and skin ulcers.
4. Discussion
Despite the rich biodiversity of the study areas, broad acceptability, and centuries-old tradition of using traditional medicines, the number of anticancer plants reported in this study is far less than expected. As it was reported by different ethnobotanical studies conducted in different parts of Ethiopia, this could be attributed to the attitude of many traditional healers to guard their indigenous medical knowledge as a family secret and hence hesitant to share with the researchers [13, 32, 73]. Justifying the lower number of female traditional healers (8, 11%) participated in this study, these studies also inferred that traditional healers usually pass their knowledge to the first son of the family.
In this study, in agreement with the studies conducted in Fiche district [35], Ghimbi district [20], and Hawassa city [17] of Ethiopia, the predominant botanical families recorded, listing over 5 plant species each, were Asteraceae, Fabaceae, and Lamiaceae. This could be due to the fact that these families are the largest in the flora of Ethiopia and Eritrea [15, 21, 143]. Moreover, cytotoxicity studies conducted on different Mexican plants reported that the highest number of plant species with both in vitro and in vivo antineoplasic activities was from these families [20].
The highest UVs recorded in this study include Aloe spp. (6), Achyranthes aspera L. (4), Albizia schimperiana (4), Sida schimperiana (4), and Brucea antidysenterica (4). The highest ICF value (0.42) recorded for “other ailments” category, in this study, suggests that informants are in agreement with the use of particular plant species to treat ailments in this category. The lowest ICF value (0) obtained was for lung, colon, cervical, and throat cancer categories. This might be due to the cultural and ecological differences of the study sites and the difficulty to pinpoint the physical symptoms of lung, colon, cervical, and throat cancer as compared to the breast and skin cancer.
The present study also revealed that RFC and CI values of some reported species are similar. However, there is a distinct difference in species ranking using each index. C. macrostachyus is placed in the first position according to both RFC and CI index. This could be due to the fact that this species is mentioned by many informants and is the most recognized plant in most study areas. Furthermore, CI value of C. macrostachyus is also high, suggesting the diversified use of the plant. V. auriculifera and C. simensis ranked next to C. macrostachyus, according to RFC index. On the other hand, D. barnimiana and Aloe spp. ranked 2nd and 3rd by CI index. It has been suggested that UV value is a good measure of use diversity, than the number of citations [144]. In agreement with this, UV value in our study is driven by species with greatest number of use rather than those cited by more informants. The Pearson correlation coefficient of −0.36, between RFC and UV, shows significant negative association between the local importance of each medicinal plant and relative importance of use of plants. This result is in contrast to previous studies that reported a significant positive correlation between RFC and UV [145, 146]. On the other hand, there is a significant positive correlation between RFC and CI (r2 = 0.74, p < 0.001) implying that their pattern matches across species. The species with larger RFC value usually have higher CI, such as Croton macrostachyus and Vernonia auriculifera.
Leaves and roots are the most commonly used plant parts in the preparation of remedies in the study districts. Similarly, other ethnobotanical studies conducted in different parts of Ethiopia also reported that leaves are the dominant plant part followed by root [16–18, 20]. The preference towards leaves may be because leaves are the main photosynthetic organs in plants and the primary reservoirs for secondary metabolites with medicinal values [36]. In contrary to other ethnobotanical studies [17, 18], where the common use of concoctions and oral route were reported, in the current study majority of the reported remedies are prepared from a single plant species and applied topically.
Comparative analysis of this study with other ethnobotanical surveys of plants used traditionally in treating and managing cancer in Ethiopia [18], Kenya [147], Cameroon [37], Nigeria [19, 38], South Africa [39], and Bangladesh [148] revealed some similarities in the plants cited in these surveys. Of the 30 plant species cited to be used in Ethiopia [18], 7 species are identified in our study: Bersama abyssinica Fresen., Brucea antidysenterica JF. Mill., Calpurnia aurea (Ait.) Benth. Dodonaea angustifolia L.f., Dorstenia barnimiana Schweinf, Kalanchoe petitiana A. Rich., and Prunus africana (Hook. f) Kalkm.
Although herbal remedies are believed by the general public to be safe [46], some research findings suggested otherwise. For instance, traditionally used Thai anticancer plants Ganoderma lucidum (Fr.) Karst., Houttuynia cordata Thunb., and Saussurea involucrata Matsum. & Koidz. were reported to cause side effects such as headache, insomnia, constipation, and diarrhea [62]. Similarly, side effects such as vomiting, diarrhea, and skin necrosis, associated with the use of traditional herbal remedies, were reported in this and other ethnobotanical studies conducted in Ethiopia [149, 150]. Few side effects reported in this study, as compared to other ethnobotanical studies conducted in Ethiopia, could be attributed to the frequent use of the topical route of administration. Nevertheless, considering the probability of underreporting adverse effects, extensive toxicological investigations should be conducted to protect the public.
In vitro cytotoxicity and antioxidant properties of some of the plants reported in our study have also been studied. Among these plants, potent cytotoxic activity was reported for knipholone anthrone isolated from Kniphofia foliosa, with IC50 value that ranges between 0.9 ± 0.1 and 3.3 ± 0.4 μg/mL [89]. Similarly, Nibret and Wink reported the cytotoxic activity of the crude extract of Acokanthera schimperi with IC50 value of 7.1 μg/mL [73]. Studies conducted on the leaves of Cineraria abyssinica [100], bark of Senna singueana [116], and bark and leaves of Rumex nepalensis [79] also revealed potent radical scavenging activity of these plants.
5. Conclusion
The present study showed that traditional healers in eleven districts of Ethiopia use different medicinal plants to manage cancer-like symptoms. Frequency of citation value ranked Croton macrostachyus Del., Clematis simensis Fresen., Dorstenia barnimiana Schweinf, Vernonia auriculifera Hiern, and Acmella caulirhiza Del. as most cited plant species in study areas. Hence, based on these findings, we are currently evaluating the in vitro antiproliferative activities of reported medicinal plant species with a higher frequency of citation against human breast adenocarcinoma (MCF-7), human uterine cervical adenocarcinoma (SiSo), human lung carcinoma (A-427), and human bladder cancer (RT-4) cell lines using crystal violate assay. However, considering the rapid disappearance of the traditional knowledge of medicinal plants and an urgent need for new anticancer agents, additional studies have to be conducted to document and scientifically validate traditionally used Ethiopian anticancer plants.
Acknowledgments
The authors wish to thank all traditional healers, individuals, data collectors, and local administrative authorities in all study districts that made this survey possible. This research was supported by the thematic research grant from the Addis Ababa University (grant number: TR/35/2015).
Data Availability
The authors declare that all data supporting the finding of this study are included in this article and its supplementary information files.
Ethical Approval
Ethical approval was obtained from Addis Ababa University, College of Health Sciences Ethics Review Board (Ref no. ERB/SOP/126/12/2015).
Consent
Each participant consented before the interview.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
A.B., E.E., and K.A. jointly conceived the study. S.T. conducted the ethnobotanical study and taxonomical identification. S.T., A.B., E.E., T.G., and K.A. enriched the draft manuscript for its intellectual content. All authors read and approved the final manuscript.
References
- 1.Zhang J., Späth S. S., Marjani S. L., Zhang W., Pan X. Characterization of cancer genomic heterogeneity by next-generation sequencing advances precision medicine in cancer treatment. Precision Clinical Medicine. 2018;1(1):29–48. doi: 10.1093/pcmedi/pby007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heng H. H., Stevens J. B., Bremer S. W., Ye K. J., Liu G., Ye C. J. The evolutionary mechanism of cancer. Journal of Cellular Biochemistry. 2014;109(6):1072–1084. doi: 10.1002/jcb.22497. [DOI] [PubMed] [Google Scholar]
- 3.Finak G., Bertos N., Pepin F., et al. Stromal gene expression predicts clinical outcome in breast cancer. Nature Medicine. 2008;14(5):518–527. doi: 10.1038/nm1764. [DOI] [PubMed] [Google Scholar]
- 4.Wu H.-C., Chang D.-K., Huang C.-T. Targeted therapy for cancer. Journal of Molecular Cancer. 2010;2(2):57–66. [Google Scholar]
- 5.Roth J. A., Fossella F., Komaki R., et al. A randomized trial comparing perioperative chemotherapy and surgery with surgery alone in resectable stage IIIA non-small-cell lung cancer. JNCI Journal of the National Cancer Institute. 1994;86(9):673–680. doi: 10.1093/jnci/86.9.673. [DOI] [PubMed] [Google Scholar]
- 6.Gregory K., Tutt A. Managing side-effects of cancer therapy. In: Gore M. E., Russell D., editors. Cancer in Primary Care. London, UK: Taylor and Francis Publishers; 2003. pp. 47–88. [DOI] [Google Scholar]
- 7.Gottesman M. M. Mechanisms of cancer drug resistance. Annual Review of Medicine. 2002;53(1):615–627. doi: 10.1146/annurev.med.53.082901.103929. [DOI] [PubMed] [Google Scholar]
- 8.Cragg G. M., Newman D. J., Snader K. M. Natural products in drug discovery and development. Journal of Natural Products. 1997;60(1):52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 9.Harvey A. Medicines from nature: are natural products still relevant to drug discovery? Trends in Pharmacological Sciences. 1999;20(5):196–198. doi: 10.1016/s0165-6147(99)01346-2. [DOI] [PubMed] [Google Scholar]
- 10.Hostettmann K., Marston A., Ndjoko K., Wolfender J.-L. The potential of African plants as a source of drugs. Current Organic Chemistry. 2000;4(10):973–1010. doi: 10.2174/1385272003375923. [DOI] [Google Scholar]
- 11.Taddese S., Asres K., Gebre-Mariam T. In vitro antimicrobial activities of some selected topically applied medicinal plants of Ethiopia. Ethiopian Pharmaceutical Journal. 2003;21:39–46. [Google Scholar]
- 12.Kelbessa S. D. E., Woldu Z., Edwards S. Some threatened endemic plants of Ethiopia. In: Edwards Z. A. S., editor. The Status of Some Plants in Parts of Tropical Africa. Addis Ababa, Ethiopia: Addis Ababa University; 1992. pp. 35–55. [Google Scholar]
- 13.Kassaye K. D., Amberbir A., Getachew B., Mussema Y. A historical overview of traditional medicine practices and policy in Ethiopia. Ethiopian Journal of Health Development. 2007;20(2):127–134. doi: 10.4314/ejhd.v20i2.10023. [DOI] [Google Scholar]
- 14.Tuasha N., Petros B., Asfaw Z. Medicinal plants used by traditional healers to treat malignancies and other human ailments in Dalle District, Sidama Zone, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2018;14(1):p. 15. doi: 10.1186/s13002-018-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chekole G., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants in the environs of Tara-gedam and Amba remnant forests of Libo Kemkem district, northwest Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2015;11(1):p. 4. doi: 10.1186/1746-4269-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Araya S., Abera B., Giday M. Study of plants traditionally used in public and animal health management in Seharti Samre district, Southern Tigray, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2015;11(1):p. 22. doi: 10.1186/s13002-015-0015-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Regassa R. Assessment of indigenous knowledge of medicinal plant practice and mode of service delivery in Hawassa city, southern Ethiopia. Journal of Medicinal Plants Research. 2013;7(9):517–535. [Google Scholar]
- 18.Agize M., Demissew S., Asfaw Z. Ethnobotany of medicinal plants in Loma and Gena bosa districts (woredas) of dawro zone, southern Ethiopia. Topclass Journal of Herbal Medicine. 2012;2(9):194–212. [Google Scholar]
- 19.Suleman S., Alemu T. A survey on utilization of ethnomedicinal plants in Nekemte town, East Wellega (Oromia), Ethiopia. Journal of Herbs, Spices & Medicinal Plants. 2012;18(1):34–57. doi: 10.1080/10496475.2011.645188. [DOI] [Google Scholar]
- 20.Abera B. Medicinal plants used in traditional medicine by Oromo people, Ghimbi district, Southwest Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 40. doi: 10.1186/1746-4269-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teklay A., Abera B., Giday M. An ethnobotanical study of medicinal plants used in Kilte Awulaelo district, Tigray region of Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2013;9(1):p. 65. doi: 10.1186/1746-4269-9-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teklehaymanot T., Giday M., Medhin G., Mekonnen Y. Knowledge and use of medicinal plants by people around Debre Libanos monastery in Ethiopia. Journal of Ethnopharmacology. 2007;111(2):271–283. doi: 10.1016/j.jep.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Ragunathan M., Abay S. M. Ethnomedicinal survey of folk drugs used in Bahirdar Zuria district, Northwestern Ethiopia. Indian Journal of Traditional Knowledge. 2009;8:281–284. [Google Scholar]
- 24.Mekuanent T., Zebene A., Solomon Z. Ethnobotanical study of medicinal plants in Chilga district, Northwestern Ethiopia. Journal of Natural Remedies. 2015;15(2):88–112. doi: 10.18311/jnr/2015/476. [DOI] [Google Scholar]
- 25.Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. Journal of Ethnopharmacology. 2009;124(1):69–78. doi: 10.1016/j.jep.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Teklehaymanot T., Giday M. Ethnobotanical study of medicinal plants used by people in Zegie Peninsula, northwestern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2007;3(1):p. 12. doi: 10.1186/1746-4269-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.CSA. Addis Ababa, Ethiopia: Central Statistical Agency; 2012. The 2007 population and housing census of Ethiopia: administrative report. [Google Scholar]
- 28.Friis I., Demissew S., Van Breugel P. Copenhagen, Denmark: Det Kongelige Danske Videnskabernes Selskab; 2010. Atlas of the potential vegetation of Ethiopia. [Google Scholar]
- 29.Tardío J., Pardo-de-Santayana M. Cultural importance indices: a comparative analysis based on the useful wild plants of southern cantabria (northern Spain)1. Economic Botany. 2008;62(1):24–39. doi: 10.1007/s12231-007-9004-5. [DOI] [Google Scholar]
- 30.Phillips O., Gentry A. H. The useful plants of Tambopata, Peru: I. Statistical hypotheses tests with a new quantitative technique. Economic Botany. 1993;47(1):15–32. doi: 10.1007/bf02862203. [DOI] [Google Scholar]
- 31.Heinrich M., Ankli A., Frei B., Weimann C., Sticher O. Medicinal plants in Mexico: healers’ consensus and cultural importance. Social Science & Medicine. 1998;47(11):1859–1871. doi: 10.1016/s0277-9536(98)00181-6. [DOI] [PubMed] [Google Scholar]
- 32.Abdela J., Engidawork E., Shibeshi W. In vivo antimalarial activity of solvent fractions of the leaves of justicia schimperiana hochst. Ex Nees against Plasmodium berghei in Mice. Ethiopian Pharmaceutical Journal. 2014;30(2):95–108. [Google Scholar]
- 33.Desta B. Ethiopian traditional herbal drugs—part III: anti-fertility activity of 70 medicinal plants. Journal of Ethnopharmacology. 1994;44(3):199–209. doi: 10.1016/0378-8741(94)01187-7. [DOI] [PubMed] [Google Scholar]
- 34.Mothana R. A., Gruenert R., Bednarski P., Lindequist U. Evaluation of the in vitro anticancer, antimicrobial and antioxidant activities of some Yemeni plants used in folk medicine. Die Pharmazie—An International Journal of Pharmaceutical Sciences. 2009;64(4):260–268. [PubMed] [Google Scholar]
- 35.Umer S., Asres K., Veeresham C. Hepatoprotective activities of two Ethiopian medicinal plants. Pharmaceutical Biology. 2010;48(4):461–468. doi: 10.3109/13880200903173593. [DOI] [PubMed] [Google Scholar]
- 36.Agisho H., Osie M., Lambore T. Traditional medicinal plants utilization, management and threats in Hadiya Zone, Ethiopia. Journal of Medicinal Plants Research. 2014;2(2):94–108. [Google Scholar]
- 37.Enyew A., Asfaw Z., Kelbessa E., Nagappan R. Status of medico-cultural commercial plants at Fiche town market, Ethiopia. International Journal of Pharmaceuticals and Health Care Research. 2013;1(4):227–236. [Google Scholar]
- 38.Kassa Z., Asfaw Z., Demissew S. Ethnobotanical study of medicinal plants used by the local people in tulu korma and its surrounding areas of ejere district, western shewa zone of oromia regional state, Ethiopia. Journal of Medicinal Plants Studies. 2016;4(2):24–47. [Google Scholar]
- 39.Zerabruk S., Yirga G. Traditional knowledge of medicinal plants in Gindeberet district, Western Ethiopia. South African Journal of Botany. 2012;78:165–169. doi: 10.1016/j.sajb.2011.06.006. [DOI] [Google Scholar]
- 40.Kefalew A., Asfaw Z., Kelbessa E. Ethnobotany of medicinal plants in ada’a district, east shewa zone of oromia regional state, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2015;11(1):p. 25. doi: 10.1186/s13002-015-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ayele T. T., Regasa M. B., Delesa D. A. Antibacterial and antagonistic activity of selected traditional medicinal plants and herbs from East Wollega zone against clinical isolated human pathogens. Science, Technology and Arts Research Journal. 2016;4(3):175–179. doi: 10.4314/star.v4i3.26. [DOI] [Google Scholar]
- 42.Bekele G., Reddy P. R. Ethnobotanical study of medicinal plants used to treat human ailments by Guji Oromo tribes in Abaya District, Borana, Oromia, Ethiopia. Universal Journal of Plant Science. 2015;3(1):1–8. [Google Scholar]
- 43.Mesfin F., Demissew S., Teklehaymanot T. An ethnobotanical study of medicinal plants in Wonago Woreda, SNNPR, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2009;5(1):p. 28. doi: 10.1186/1746-4269-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giday M., Teklehaymanot T., Animut A., Mekonnen Y. Medicinal plants of the shinasha, agew-awi and Amhara peoples in northwest Ethiopia. Journal of Ethnopharmacology. 2007;110(3):516–525. doi: 10.1016/j.jep.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Eshete M. A., Kelbessa E., Dalle G. Ethnobotanical study of medicinal plants in guji agro-pastoralists, blue Hora district of Borana zone, Oromia region, Ethiopia. Journal of Medicinal Plants Studies. 2016;4(2):170–184. [Google Scholar]
- 46.Yineger H., Kelbessa E., Bekele T., Lulekal E. Plants used in traditional management of human ailments at Bale mountains national park, southeastern Ethiopia. Journal of Medicinal Plants Research. 2008;2(6):132–153. [Google Scholar]
- 47.Megersa M., Asfaw Z., Kelbessa E., Beyene A., Woldeab B. An ethnobotanical study of medicinal plants in Wayu Tuka district, east Welega zone of oromia regional state, West Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2013;9(1):p. 68. doi: 10.1186/1746-4269-9-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belayneh A., Asfaw Z., Demissew S., Bussa N. F. Medicinal plants potential and use by pastoral and agro-pastoral communities in Erer Valley of Babile Wereda, Eastern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2012;8(1):p. 42. doi: 10.1186/1746-4269-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wondimu T., Asfaw Z., Kelbessa E. Ethnobotanical study of medicinal plants around ‘Dheeraa’ town, Arsi Zone, Ethiopia. Journal of Ethnopharmacology. 2007;112(1):152–161. doi: 10.1016/j.jep.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 50.Yineger H., Yewhalaw D., Teketay D. Ethnomedicinal plant knowledge and practice of the Oromo ethnic group in southwestern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2008;4(1):p. 11. doi: 10.1186/1746-4269-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Asamenew G., Bisrat D., Mazumder A., Asres K. In vitro antimicrobial and antioxidant activities of anthrone and chromone from the latex of Aloe harlana Reynolds. Phytotherapy Research. 2011;25(12):1756–1760. doi: 10.1002/ptr.3482. [DOI] [PubMed] [Google Scholar]
- 52.Dagne E., Bisrat D., Viljoen A., Van Wyk B.-E. Chemistry of Aloe species. Current Organic Chemistry. 2000;4(10):1055–1078. doi: 10.2174/1385272003375932. [DOI] [Google Scholar]
- 53.Deressa T., Mekonnen Y., Animut A. In vivo anti-malarial activities of Clerodendrum myricoides, Dodonea angustifolia and Aloe debrana against Plasmodium berghei. Ethiopian Journal of Health Development. 2010;24:1. doi: 10.4314/ejhd.v24i1.62941. [DOI] [Google Scholar]
- 54.Mesfin A., Giday M., Animut A., Teklehaymanot T. Ethnobotanical study of antimalarial plants in Shinile district, Somali region, Ethiopia, and in vivo evaluation of selected ones against Plasmodium berghei. Journal of Ethnopharmacology. 2012;139(1):221–227. doi: 10.1016/j.jep.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 55.d’Avigdor E., Wohlmuth H., Asfaw Z., Awas T. The current status of knowledge of herbal medicine and medicinal plants in Fiche, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 38. doi: 10.1186/1746-4269-10-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Belayneh A., Bussa N. F. Ethnomedicinal plants used to treat human ailments in the prehistoric place of Harla and Dengego valleys, eastern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 18. doi: 10.1186/1746-4269-10-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yirga G. Assessment of indigenous knowledge of medicinal plants in central zone of Tigray, northern Ethiopia. African Journal of Plant Science. 2010;4(1):6–11. [Google Scholar]
- 58.Zenebe G., Zerihun M., Solomon Z. An ethnobotanical study of medicinal plants in Asgede Tsimbila district, northwestern Tigray, northern Ethiopia. Ethnobotany Research and Applications. 2012;10:305–320. doi: 10.17348/era.10.0.305-320. [DOI] [Google Scholar]
- 59.Teklehaymanot T., Giday M. Ethnobotanical study of wild edible plants of kara and kwego semi-pastoralist people in lower omo river valley, debub omo zone, SNNPR, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2010;6(1):p. 23. doi: 10.1186/1746-4269-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shibeshi W., Makonnen E., Zerihun L., Debella A. Effect of Achyranthes aspera L. on fetal abortion, uterine and pituitary weights, serum lipids and hormones. African Health Sciences. 2006;6(2):108–112. doi: 10.5555/afhs.2006.6.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fikru A., Makonnen E., Eguale T., Debella A., Abie Mekonnen G. Evaluation of in vivo wound healing activity of methanol extract of Achyranthes aspera L. Journal of Ethnopharmacology. 2012;143(2):469–474. doi: 10.1016/j.jep.2012.06.049. [DOI] [PubMed] [Google Scholar]
- 62.Enyew A., Asfaw Z., Kelbessa E., Nagappan R. Ethnobotanical study of traditional medicinal plants in and around Fiche district, Central Ethiopia. Current Research Journal of Biological Sciences. 2014;6(4):154–167. doi: 10.19026/crjbs.6.5515. [DOI] [Google Scholar]
- 63.Giday M., Asfaw Z., Woldu Z. Ethnomedicinal study of plants used by Sheko ethnic group of Ethiopia. Journal of Ethnopharmacology. 2010;132(1):75–85. doi: 10.1016/j.jep.2010.07.046. [DOI] [PubMed] [Google Scholar]
- 64.Lulekal E., Asfaw Z., Kelbessa E., Van Damme P. Ethnoveterinary plants of Ankober district, north Shewa zone, Amhara region, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 21. doi: 10.1186/1746-4269-10-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mesfin F., Seta T., Assefa A. An ethnobotanical study of medicinal plants in Amaro Woreda, Ethiopia. Ethnobotany Research and Applications. 2014;12:341–354. doi: 10.17348/era.12.0.341-354. [DOI] [Google Scholar]
- 66.Barnes J., Anderson L. A., Phillipson J. D., Newall C. A. Herbal Medicines. London, UK: Pharmaceutical Press London; 2007. [Google Scholar]
- 67.Jamil S. S., Nizami Q., Salam M. Centella asiatica (Linn.) Urban: a review. Natural Product Radiance. 2007;6(2):158–170. [Google Scholar]
- 68.Yoshida M., Fuchigami M., Nagao T., et al. Antiproliferative constituents from Umbelliferae plants VII: active triterpenes and rosmarinic acid from Centella asiatica. Biological & Pharmaceutical Bulletin. 2005;28(1):173–175. doi: 10.1248/bpb.28.173. [DOI] [PubMed] [Google Scholar]
- 69.Bunpo P., Kataoka K., Arimochi H., et al. Inhibitory effects of Centella asiatica on azoxymethane-induced aberrant crypt focus formation and carcinogenesis in the intestines of F344 rats. Food and Chemical Toxicology. 2004;42(12):1987–1997. doi: 10.1016/j.fct.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 70.Kidane B., van Andel T., van der Maesen L., Asfaw Z. Use and management of traditional medicinal plants by Maale and Ari ethnic communities in southern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 46. doi: 10.1186/1746-4269-10-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tolossa K., Debela E., Athanasiadou S., Tolera A., Ganga G., Houdijk J. G. Ethno-medicinal study of plants used for treatment of human and livestock ailments by traditional healers in south Omo, southern Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2013;9(1):p. 32. doi: 10.1186/1746-4269-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Giday M., Asfaw Z., Woldu Z. Medicinal plants of the Meinit ethnic group of Ethiopia: an ethnobotanical study. Journal of Ethnopharmacology. 2009;124(3):513–521. doi: 10.1016/j.jep.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 73.Nibret E., Wink M. Trypanocidal and cytotoxic effects of 30 Ethiopian medicinal plants. Zeitschrift für Naturforschung C. 2011;66(11-12):0541–0546. doi: 10.1515/znc-2011-11-1202. [DOI] [PubMed] [Google Scholar]
- 74.Gebre-Mariam T., Neubert R., Schmidt P., Wutzler P., Schmidtke M. Antiviral activities of some Ethiopian medicinal plants used for the treatment of dermatological disorders. Journal of Ethnopharmacology. 2006;104(1-2):182–187. doi: 10.1016/j.jep.2005.08.071. [DOI] [PubMed] [Google Scholar]
- 75.Tadeg H., Mohammed E., Asres K., Gebre-Mariam T. Antimicrobial activities of some selected traditional Ethiopian medicinal plants used in the treatment of skin disorders. Journal of Ethnopharmacology. 2005;100(1-2):168–175. doi: 10.1016/j.jep.2005.02.031. [DOI] [PubMed] [Google Scholar]
- 76.Mohammed T., Erko B., Giday M. Evaluation of antimalarial activity of leaves of Acokanthera schimperi and Croton macrostachyus against Plasmodium berghei in Swiss albino mice. BMC Complementary and Alternative Medicine. 2014;14(1):p. 314. doi: 10.1186/1472-6882-14-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abebe D., Ayehu A. Medicinal Plants and Enigmatic Health Practices of Northern Ethiopia. Addis Ababa, Ethiopia: Berhanina Selam Printing Press; 1993. [Google Scholar]
- 78.Meaza G., Tadesse B., Maria A. S., Piero B., Gidey Y. Traditional medicinal plants used by Kunama ethnic group in northern Ethiopia. Journal of Medicinal Plants Research. 2015;9(15):494–509. doi: 10.5897/jmpr2014.5681. [DOI] [Google Scholar]
- 79.Tauchen J., Doskocil I., Caffi C., et al. In vitro antioxidant and anti-proliferative activity of Ethiopian medicinal plant extracts. Industrial Crops and Products. 2015;74:671–679. doi: 10.1016/j.indcrop.2015.05.068. [DOI] [Google Scholar]
- 80.Birhanu Z. Ethno-botanical survey on medicinal plants used by ethnic groups of Denbia district, north-western Ethiopia. Journal of Natural Remedies. 2011;11(2):119–123. [Google Scholar]
- 81.Seyoum G., Zerihun G. An ethnobotanical study of medicinal plants in Debre Libanos Wereda, Central Ethiopia. African Journal of Plant Science. 2014;8(7):366–379. doi: 10.5897/ajps2013.1041. [DOI] [Google Scholar]
- 82.Heli H., Amani M., Moosavi-Movahedi A. A., Jabbari A., Floris G., Mura A. Electroactive centers in Euphorbia latex and lentil seedling amine oxidases. Bioscience, Biotechnology, and Biochemistry. 2008;72(1):29–36. doi: 10.1271/bbb.70299. [DOI] [PubMed] [Google Scholar]
- 83.Setty S. R., Quereshi A. A., Swamy A. V., et al. Hepatoprotective activity of Calotropis procera flowers against paracetamol-induced hepatic injury in rats. Fitoterapia. 2007;78(7-8):451–454. doi: 10.1016/j.fitote.2006.11.022. [DOI] [PubMed] [Google Scholar]
- 84.Roy S., Sehgal R., Padhy B. M., Kumar V. L. Antioxidant and protective effect of latex of Calotropis procera against alloxan-induced diabetes in rats. Journal of Ethnopharmacology. 2005;102(3):470–473. doi: 10.1016/j.jep.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 85.Kamath J. V., Rana A. C. Preliminary study on antifertility activity of Calotropis procera roots in female rats. Fitoterapia. 2002;73(2):111–115. doi: 10.1016/s0367-326x(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 86.Sayed A. E.-D. H., Mohamed N. H., Ismail M. A., Abdel-Mageed W. M., Shoreit A. A. M. Antioxidant and antiapoptotic activities of Calotropis procera latex on Catfish (Clarias gariepinus) exposed to toxic 4-nonylphenol. Ecotoxicology and Environmental Safety. 2016;128:189–194. doi: 10.1016/j.ecoenv.2016.02.023. [DOI] [PubMed] [Google Scholar]
- 87.Flatie T., Gedif T., Asres K., Gebre-Mariam T. Ethnomedical survey of Berta ethnic group Assosa zone, Benishangul-Gumuz regional state, mid-west Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2009;5(1):p. 14. doi: 10.1186/1746-4269-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wube A. A., Bucar F., Asres K., Gibbons S., Rattray L., Croft S. L. Antimalarial compounds from Kniphofia foliosa roots. Phytotherapy Research. 2005;19(6):472–476. doi: 10.1002/ptr.1635. [DOI] [PubMed] [Google Scholar]
- 89.Habtemariam S. Knipholone anthrone from Kniphofia foliosa induces a rapid onset of necrotic cell death in cancer cells. Fitoterapia. 2010;81(8):1013–1019. doi: 10.1016/j.fitote.2010.06.021. [DOI] [PubMed] [Google Scholar]
- 90.Habtemariam S. Antioxidant activity of Knipholone anthrone. Food Chemistry. 2007;102(4):1042–1047. doi: 10.1016/j.foodchem.2006.06.040. [DOI] [Google Scholar]
- 91.Crouch N. R., Langlois A., Mulholland D. A., Nair J. J., Houghton P. A novel alkylamide from the leaves of Acmella caulirhiza (Asteraceae), a traditional surface analgesic. South African Journal of Botany. 2005;71(2):228–230. doi: 10.1016/s0254-6299(15)30137-x. [DOI] [Google Scholar]
- 92.Owuor B. O., Ochanda J. O., Kokwaro J. O., et al. In vitro antiplasmodial activity of selected Luo and Kuria medicinal plants. Journal of Ethnopharmacology. 2012;144(3):779–781. doi: 10.1016/j.jep.2012.09.045. [DOI] [PubMed] [Google Scholar]
- 93.Tariku Y., Hymete A., Hailu A., Rohloff J. In vitro evaluation of antileishmanial activity and toxicity of essential oils of Artemisia absinthium and Echinops kebericho. Chemistry & Biodiversity. 2011;8(4):614–623. doi: 10.1002/cbdv.201000331. [DOI] [PubMed] [Google Scholar]
- 94.Nibret E., Wink M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine. 2010;17(5):369–374. doi: 10.1016/j.phymed.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 95.Burits M., Asres K., Bucar F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phytotherapy Research. 2001;15(2):103–108. doi: 10.1002/ptr.691. [DOI] [PubMed] [Google Scholar]
- 96.Zhu X. X., Yang L., Li Y. J., et al. Effects of sesquiterpene, flavonoid and coumarin types of compounds from Artemisia annua L. on production of mediators of angiogenesis. Pharmacological Reports. 2013;65(2):410–420. doi: 10.1016/s1734-1140(13)71016-8. [DOI] [PubMed] [Google Scholar]
- 97.Zhai D.-D., Supaibulwatana K., Zhong J.-J. Inhibition of tumor cell proliferation and induction of apoptosis in human lung carcinoma 95-D cells by a new sesquiterpene from hairy root cultures of Artemisia annua. Phytomedicine. 2010;17(11):856–861. doi: 10.1016/j.phymed.2010.02.008. [DOI] [PubMed] [Google Scholar]
- 98.Zheng G.-Q. Cytotoxic terpenoids and flavonoids from Artemisia annua. Planta Medica. 1994;60(1):54–57. doi: 10.1055/s-2006-959408. [DOI] [PubMed] [Google Scholar]
- 99.Teshome T., Sintayehu B., Yohannes H., et al. Radical scavenging activity and preliminary phytochemical screening on aerial part extracts of Cineraria abyssinica sch. bip. Journal of Pharmacognosy and Phytochemistry. 2015;3(6):239–243. [Google Scholar]
- 100.Sintayehu B., Asres K., Raghavendra Y. Radical scavenging activities of the leaf extracts and a flavonoid glycoside isolated from Cineraria abyssinica Sch. Bip. Exa. Rich. Journal of Applied Pharmaceutical Science. 2012;2(4):44–49. doi: 10.7324/japs.2012.2407. [DOI] [Google Scholar]
- 101.Cos P., Hermans N., De Bruyne T., et al. Further evaluation of Rwandan medicinal plant extracts for their antimicrobial and antiviral activities. Journal of Ethnopharmacology. 2002;79(2):155–163. doi: 10.1016/s0378-8741(01)00362-2. [DOI] [PubMed] [Google Scholar]
- 102.Asres K., Bucar F., Kartnig T., Witvrouw M., Pannecouque C., De Clercq E. Antiviral activity against human immunodeficiency virus type 1 (HIV-1) and type 2 (HIV-2) of ethnobotanically selected Ethiopian medicinal plants. Phytotherapy Research. 2001;15(1):62–69. doi: 10.1002/1099-1573(200102)15:1<62::aid-ptr956>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 103.Albejo B., Endale M., Kibret B., Anza M. Phytochemical investigation and antimicrobial activity of leaves extract of Vernonia auriculifera Hiern. Journal of Pharmacy & Pharmacognosy Research. 2015;3(6):141–147. [Google Scholar]
- 104.Ibrahim J., Ajaegbu V. C., Egharevba H. O. Pharmacognostic and phytochemical analysis of Commelina benghalensis L. Ethnobotanical Leaflets. 2010;2010(5):p. 7. [Google Scholar]
- 105.Hasan S., Hossain M., Akter R., et al. Sedative and anxiolytic effects of different fractions of the Commelina benghalensis Linn. Drug Discoveries & Therapeutics. 2009;3(5) [PubMed] [Google Scholar]
- 106.Mekonnen A., Sidamo T., Asres K., Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. Journal of Ethnopharmacology. 2013;145(2):638–646. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 107.Abebe D., Debella A., Urga K. Medicinal Plants and other Useful Plants of Ethiopia. Addis Ababa, Ethiopia: Camerapix Publishers International; 2013. [Google Scholar]
- 108.Bussmann R. W., Swartzinsky P., Worede A., Evangelista P. Plant use in odo-bulu and demaro, Bale region, Ethiopia. Journal of Ethnobiology and Ethnomedicine. 2011;7(1):p. 28. doi: 10.1186/1746-4269-7-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Birhanu Z. Traditional use of medicinal plants by the ethnic groups of Gondar Zuria district, north-western Ethiopia. Journal of Natural Remedies. 2011;13(1):46–53. [Google Scholar]
- 110.Limenih Y., Umer S., Wolde-Mariam M. Ethnobotanical study on traditional medicinal plants in Dega damot woreda, Amhara region, north Ethiopia. International Journal of Research in Pharmacy and Chemistry. 2015;5:258–273. [Google Scholar]
- 111.Belay G., Tariku Y., Kebede T., Hymete A., Mekonnen Y. Ethnopharmacological investigations of essential oils isolated from five Ethiopian medicinal plants against eleven pathogenic bacterial strains. Phytopharmacology. 2011;1(5):133–143. [Google Scholar]
- 112.Almehdar H., Abdallah H. M., Osman A.-M. M., Abdel-Sattar E. A. In vitro cytotoxic screening of selected Saudi medicinal plants. Journal of Natural Medicines. 2012;66(2):406–412. doi: 10.1007/s11418-011-0589-8. [DOI] [PubMed] [Google Scholar]
- 113.Asres K., Gibbons W. A., Phillipson J. D., Mascagni P. Alkaloids of Ethiopian Calpurnia aurea subsp. aurea. Phytochemistry. 1986;25(6):1443–1447. doi: 10.1016/s0031-9422(00)81306-0. [DOI] [Google Scholar]
- 114.Asres K., Phillipson J., Mascagni P. Two novel minor alkaloids from Ethiopian Calpurnia aurea sp.aurea. Planta Medica. 1986;52(4):302–304. doi: 10.1055/s-2007-969159. [DOI] [PubMed] [Google Scholar]
- 115.Asres K., Sporer F., Wink M. Patterns of pyrrolizidine alkaloids in 12 Ethiopian Crotalaria species. Biochemical Systematics and Ecology. 2004;32(10):915–930. doi: 10.1016/j.bse.2004.03.004. [DOI] [Google Scholar]
- 116.Gebrelibanos M., Asres K., Veeresham C. In vitro radical scavenging activity of the leaf and bark extracts of Senna singueana (Del). Lock. Ethiopian Pharmaceutical Journal. 2008;25:77–84. doi: 10.4314/epj.v25i2.35121. [DOI] [Google Scholar]
- 117.Tchoumbougnang F., Zollo P. H., Dagne E., Mekonnen Y. In Vivo antimalarial activity of essential oils from Cymbopogon citratus and Ocimum gratissimumon mice infected with Plasmodium berghei. Planta Medica. 2005;71(1):20–23. doi: 10.1055/s-2005-837745. [DOI] [PubMed] [Google Scholar]
- 118.Asres K., Asfaha H., Mazumder A., Bucar F. Leaf essential oils of Salvia nilotica and Salvia schimperi: their antimicrobial and antioxidant activities. Ethiopian Pharmaceutical Journal. 2008;26(1):49–58. doi: 10.4314/epj.v26i1.35132. [DOI] [Google Scholar]
- 119.Engeda D., Geremew B., Gulelat D. H., H P. V. R. Antioxidant and -amylase inhibition activities in vitro of various solvent extracts of Thymus schimperi Ronniger. Journal of Medicinal Plants Research. 2015;9(15):515–524. doi: 10.5897/jmpr2014.5431. [DOI] [Google Scholar]
- 120.Geyid A., Abebe D., Debella A., et al. Screening of some medicinal plants of Ethiopia for their anti-microbial properties and chemical profiles. Journal of Ethnopharmacology. 2005;97(3):421–427. doi: 10.1016/j.jep.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 121.Kathiriya A., Das K., Kumar E., Mathai K. Evaluation of antitumor and antioxidant activity of oxalis corniculata linn: against ehrlich ascites carcinoma on mice. Iranian Journal of Cancer Prevention. 2010;3:4. [Google Scholar]
- 122.Kasimala B. B., Anna V. R., Mallu U. R. Reverse phase liquid chromatographic method for the simultaneous estimation of antibiotic drugs: metronidazole, nalidixic acid, tinidazole and norfloxacin. Der Pharmacia Lettre. 2014;6:411–419. [Google Scholar]
- 123.Vasas A., Orbán-Gyapai O., Hohmann J. The genus rumex: review of traditional uses, phytochemistry and pharmacology. Journal of Ethnopharmacology. 2015;175:198–228. doi: 10.1016/j.jep.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 124.Tadele A., Asres K., Melaku D., Mekonnen W. In vivo anti-inflammatory and antinociceptive activities of the leaf extracts of Clematis simensis Fresen. Ethiopian Pharmaceutical Journal. 2010;27:33–41. doi: 10.4314/epj.v27i1.51117. [DOI] [Google Scholar]
- 125.Songue J., Kouam E., Dongo E., Mpondo T., White R. Chemical constituents from stem bark and roots of Clausena anisata. Molecules. 2012;17(11):13673–13686. doi: 10.3390/molecules171113673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Abebaw M., Mishra B., Gelayee D. A. Evaluation of anti-ulcer activity of the leaf extract of. Journal of Experimental Pharmacology. 2017;9:1–11. doi: 10.2147/jep.s125383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rached W., Calhelha R. C., Fernandes Â., et al. Phytochemical characterization and bioactive properties of Osyris quadripartita Salzm. ex Decne. leaves from Algeria. RSC Advances. 2016;6(76):72768–72776. doi: 10.1039/c6ra11787b. [DOI] [Google Scholar]
- 128.Riaz T., Abbasi M. A., Shahzadi T., Ajaib M., Khan K. M. Phytochemical screening, free radical scavenging, antioxidant activity and phenolic content of Dodonaea viscosa. Journal of the Serbian Chemical Society. 2012;77:1. doi: 10.2298/jsc110621183r. [DOI] [Google Scholar]
- 129.Teshome K., Gebre‐Mariam T., Asres K., Engidawork E. Toxicity studies on dermal application of plant extract of Dodonaea viscosa used in Ethiopian traditional medicine. Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives. 2010;24(1):60–69. doi: 10.1002/ptr.2869. [DOI] [PubMed] [Google Scholar]
- 130.Amuamuta A., Mekonnen Z., Gebeyehu E. Traditional therapeutic uses and phytochemical screening of some selected indigenous medicinal plants from Northwest Ethiopia. African Journal of Pharmacology and Therapeutics. 2015;4(3) [Google Scholar]
- 131.Kefe A., Giday M., Mamo H., Erko B. Antimalarial properties of crude extracts of seeds of Brucea antidysenterica and leaves of Ocimum lamiifolium. BMC Complementary and Alternative Medicine. 2016;16(1):p. 118. doi: 10.1186/s12906-016-1098-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Habtemariam S., Gray A. I. Withanolides from the roots of Discopodium penninervium. Planta Medica. 1998;64(3):275–276. doi: 10.1055/s-2006-957426. [DOI] [PubMed] [Google Scholar]
- 133.Habtemariam S., Gray A. I., Waterman P. G. 16-oxygenated withanolides from the leaves of Discopodium penninervium. Phytochemistry. 1993;34(3):807–811. doi: 10.1016/0031-9422(93)85363-v. [DOI] [Google Scholar]
- 134.Habtemariam S., Skelton B. W., Waterman P. G., White A. H. 17-Epiacnistin-A, a further withanolide from the leaves of discopodium p enninervium. Journal of Natural Products. 2000;63(4):512–513. doi: 10.1021/np990446x. [DOI] [PubMed] [Google Scholar]
- 135.Wube A. A., Wenzig E.-M., Gibbons S., Asres K., Bauer R., Bucar F. Constituents of the stem bark of Discopodium penninervium and their LTB4 and COX-1 and-2 inhibitory activities. Phytochemistry. 2008;69(4):982–987. doi: 10.1016/j.phytochem.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Habtemariam S. Cytotoxicity and immunosuppressive activity of withanolides from Discopodium penninervium. Planta Medica. 1997;63(1):15–17. doi: 10.1055/s-2006-957594. [DOI] [PubMed] [Google Scholar]
- 137.Ferrari J., Terreaux C., Sahpaz S., Msonthi J. D., Wolfender J.-L., Hostettmann K. Benzophenone glycosides from Gnidia involucrata. Phytochemistry. 2000;54(8):883–889. doi: 10.1016/s0031-9422(00)00046-7. [DOI] [PubMed] [Google Scholar]
- 138.Imbenzi P. S., He Y.-Z., Yan Z.-X., Osoro E. K., Cheplogoi P. K. Chemical constituents in extracts from leaves of Lantana trifolia and their in vitro anti-oxidative activity. Chinese Herbal Medicines. 2014;6(3):242–246. doi: 10.1016/s1674-6384(14)60035-6. [DOI] [Google Scholar]
- 139.Valerino A., González T., Spengler I. Bio-directed phytochemical study of the allelopathic activity of Lantana trifolia L. leaves. Part I. Proceedings of the XVII Congreso de la Asociación Latinoamericana de Malezas (ALAM) I Congreso Iberoamericano de Ciencia de las Malezas, IV Congreso Nacional de Ciencia de Malezas; November 2005; Matanzas, Cuba. Asociación Latinoamericana de Malezas (ALAM); pp. 652–660. [Google Scholar]
- 140.Abegaz B., Asfaw N., Lwande W. Constituents of the essential oils from wild and cultivated Lippia adoensis Hochst. ex Walp. Journal of Essential Oil Research. 1993;5(5):487–491. doi: 10.1080/10412905.1993.9698268. [DOI] [Google Scholar]
- 141.Asres K., Bucar F. Lippia adoensis var. adoensis: studies on the essential oil composition and antioxidant activity. Ethiopian Pharmaceutical Journal. 2002;20:32–38. [Google Scholar]
- 142.Debella A., Makonnen E., Abebe D., Teka F., Kidanemariam A. Pain management in mice using the aqueous and ethanol extracts of four medicinal plants. East African Medical Journal. 2004;80(8):435–439. doi: 10.4314/eamj.v80i8.8737. [DOI] [PubMed] [Google Scholar]
- 143.Tesfaye A., Makonnen E., Gedamu S. Hypoglycemic and antihyperglycemic activity of aqueous extract of Justicia Schimperiana leaves in normal and streptozotocin-induced diabetic mice. International Journal of Pharma Sciences and Research. 2016;7(2):110–113. [Google Scholar]
- 144.Da Silva V. A., Andrade L. D. H. C., De Albuquerque U. P. Revising the cultural significance index: the case of the Fulni-ô in northeastern Brazil. Field Methods. 2006;18(1):98–108. doi: 10.1177/1525822x05278025. [DOI] [Google Scholar]
- 145.Ahmad K. S., Hamid A., Nawaz F., et al. Ethnopharmacological studies of indigenous plants in Kel village, Neelum Valley, Azad Kashmir, Pakistan. Journal of Ethnobiology and Ethnomedicine. 2017;13(1):p. 68. doi: 10.1186/s13002-017-0196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bano A., Ahmad M., Hadda T. B., et al. Quantitative ethnomedicinal study of plants used in the skardu valley at high altitude of Karakoram-Himalayan range, Pakistan. Journal of Ethnobiology and Ethnomedicine. 2014;10(1):p. 43. doi: 10.1186/1746-4269-10-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Birhanu Z., Endale A., Shewamene Z. An ethnomedicinal investigation of plants used by traditional healers of Gondar town, north-western Ethiopia. Journal of Medicinal Plants Studies. 2015;3(2):36–43. [Google Scholar]
- 148.Kebu B., Ensermu K., Zemede A. Indigenous medicinal utilization, management and threats in Fentale area, Eastern Shewa, Ethiopia. Ethiopian Journal of Biological Sciences. 2015;3(1):37–58. [Google Scholar]
- 149.Ryding O. Lamiaceae. In: Hedberg E. K. I., Edwards S., Demissew S., Persson E., editors. Flora of Ethiopia and Eritrea. Addis Ababa, Ethiopia: The National Herbarium, Addis Ababa University; 2006. pp. 516–604. [Google Scholar]
- 150.Tadesse M. Asteraceae (compositae) In: Hedberg I. F. I., Edwards S., editors. Flora of Ethiopia and Eritrea. Addis Ababa, Ethiopia: Addis Ababa and Uppsala: The National Herbarium, Addis Ababa University and Uppsala University; 2004. pp. 222–223. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors declare that all data supporting the finding of this study are included in this article and its supplementary information files.


