Abstract
Respiratory disease is a leading cause of mortality in patients with osteogenesis imperfecta (OI), a connective tissue disease that causes severely reduced bone mass and is most commonly caused by dominant mutations in type I collagen genes. Previous studies proposed that impaired respiratory function in OI patients was secondary to skeletal deformities; however, recent evidence suggests the existence of a primary lung defect. Here, we analyzed the lung phenotype of Crtap knockout (KO) mice, a mouse model of recessive OI. While we confirm changes in the lung parenchyma that are reminiscent of emphysema, we show that CrtapKO lung fibroblasts synthesize type I collagen with altered posttranslation modifications consistent with those observed in bone and skin. Unrestrained whole body plethysmography showed a significant decrease in expiratory time, resulting in an increased ratio of inspiratory time over expiratory time and a concomitant increase of the inspiratory duty cycle in CrtapKO compared with WT mice. Closed-chest measurements using the forced oscillation technique showed increased respiratory system elastance, decreased respiratory system compliance, and increased tissue damping and elasticity in CrtapKO mice compared with WT. Pressure-volume curves showed significant differences in lung volumes and in the shape of the curves between CrtapKO mice and WT mice, with and without adjustment for body weight. This is the first evidence that collagen defects in OI cause primary changes in lung parenchyma and several respiratory parameters and thus negatively impact lung function.
Keywords: collagen, Crtap, lung, osteogenesis imperfecta, respiratory function
INTRODUCTION
The skeletal dysplasia known as osteogenesis imperfecta (OI or brittle bone disease) is the most commonly inherited bone fragility disorder and is characterized by low bone mass and high fracture incidence (44). Its clinical presentation is often heterogeneous, and patients can have other skeletal abnormalities, including growth deficiency, deformity of bones, distortions of the chest, scoliosis, kyphosis, joint and ligamentous laxity (18, 36, 52). Because of the wide spectrum of severity, OI was originally classified into four groups: a mild form of the disease (OI type I), a perinatal lethal form (OI type II), a severe and progressively deforming type (OI type III), and a moderately severe disease (OI type IV) (51). The most common causes of OI are dominant mutations in COL1A1 and COL1A2 genes, encoding the α1- and α2-chains of type I collagen, but several rarer, recessive, and X-linked inherited forms of the disease have also been identified (10, 35). These are often due to loss of function of proteins involved in type I collagen processing. Because type I collagen is the most abundant collagen in the human body and is expressed in all connective tissues, the disease is systemic, and extra skeletal features, such as dentinogenesis imperfecta, blue sclerae, hearing impairment, skin laxity, and cardiorespiratory issues, can be variably associated with OI (36, 44).
Importantly, respiratory distress at birth and poor respiratory function and increased vulnerability to lung infections, which can lead to fatal pneumonia in adulthood, represent significant issues in patients with moderate to severe OI (37, 50). A recent study on 687 OI patients who were followed for more than 35 yr, reported that patients with OI are at increased risk of death due to respiratory disease compared with the general population (16). The conventional view of restrictive lung function in cases of severe OI is that it is largely due to abnormalities of the chest wall caused by numerous factors, including kyphoscoliosis, fractures of the ribs, and even short stature, causing diaphragm restriction (49). These restrictions ultimately result in reduced alveolar ventilation due to lung compression and lead to reduction in mucociliary clearance and favor infection from colonization of microorganisms (49). Despite the fact that deaths due to pulmonary dysfunction can be attributed to secondary effects of the skeletal deformities, many patients with milder forms of OI that lack malformations of the chest cavity are still at a higher risk of death due to respiratory complications (64). Case studies of lethal OI with lung hypoplasia have suggested that abnormal collagen might be directly causal to the death of these patients (50, 57); moreover, moderate to severe OI patients suffering respiratory insufficiency at birth can be further negatively impacted by the standard pharmacological treatment with bisphosphonates, which elicit a flu-like syndrome characterized by pulmonary edema and respiratory distress (1, 41).
Mouse models of OI also exhibit lung alterations in addition to the skeletal phenotype. For instance, the Aga2 mouse model caused by a dominant frameshift mutation in the proα1(I) C-propeptide domain shows pathological changes in lung and cardiac tissue that result in lethality (58). These mice mimic the symptoms of pediatric patients with type III and IV OI who harbor similar type I collagen mutations and have decreased respiratory function over time despite absence of scoliosis (58). The Crtap knockout (KO) mice lack a functional prolyl 3-hydroxylation complex in the ER, which is essential for type I collagen posttranslational modification (prolyl 3-hydroxylation) and folding (6, 8, 39). Biallelic CRTAP mutations in humans cause severe to lethal OI, and newborns frequently succumb to respiratory distress (40). The CrtapKO mice represent a model of recessive OI and were shown to also have altered lung histology, including increased alveolar space and thinning of the alveolar walls (5). CrtapKO mice have a global increase in TGF-β signaling, and inhibition of this pathway rescued their bone mass but only partially rescued the lung phenotype (22). Another mouse model of severe dominant OI with a type I collagen mutation (Col1a1Jrt/+) was recently shown to have contractile weakness of the diaphragm and pulmonary airspace enlargement, but these did not improve after anti-TGF-β antibody treatment (4).
Although the lung extracellular matrix (ECM), in addition to providing structural support for cells, is critical for the regulation of organogenesis, homeostasis, and injury-repair responses (65), no studies have addressed if and how abnormal collagen contributes to respiratory disease in OI. Therefore, the aims of this study were to assess lung parenchyma changes and identify specific collagen defects in the lung of CrtapKO mice, and determine if these alterations modify their respiratory pattern and/or respiratory mechanics.
MATERIALS AND METHODS
Mice
All animal work performed in this study was conducted in accordance to local, state, and U.S. federal regulations. CrtapKO mice were generated previously (39), maintained in a mixed C57BL6/J;129/SvEv genetic background, and genotyped by a PCR protocol using the primers forward 5′-TGACCGCTTCCTCGTGC-3′ and reverse 5′-CCCGCCTATCACCAACC-3′ for detecting the mutant allele, and forward 5′-GGCCAATGACCTCCCGAAG-3′ and reverse 5′-AACTTCGGGGTAAAGCCAGAG-3′ for the wild-type (WT) allele. The products of the reaction are ~500 base pairs (bp) for the mutant allele and 180 bp for the WT allele (39). Mice were housed in a pathogen-free facility, with unlimited access to water and standard rodent chow and with a 12:12-h light-dark cycle. The University of Arkansas for Medical Sciences Institutional Animal Care and Use Committee approved the animal protocol (AUP no. 3845).
Cell Culture and Tissue Histology
Primary murine lung fibroblasts were grown in Dulbecco’s modified Eagle’s medium/Ham’s F-12 (DMEM-F12 50:50 mix) with l-glutamine and 15 mM HEPES and supplemented with fetal bovine serum (FBS), either 10 or 15% with the addition of 100 U/mL penicillin and 100 mg/mL streptomycin. They were harvested from 5-day-old pups as follows: lungs were dissected under sterile conditions, collected into wells of a six-well plate containing 1 mL of digestion media, and cut into small pieces using two scalpels. The minced tissue was then placed into a 15 mL Falcon with 2 mL of digestion media [collagenase A (1 mg/mL, catalog no. 0103578001; Roche), trypsin 0.25% 1× (catalog no. SH3004201; Fisher Scientific Hyclone) and DMEM-F12 50:50/GLN serum free (catalog no. 45000–350; Corning-VWR)], set in a water bath a 37C° for 90 min, and vortex gently every 30 min. After the digestion, cells were spun down, resuspended into 2 mL of DMEM-F12 complete with 15% FBS, and plated into a well of a six-well plate. The next day, cells were washed with 1× PBS two times and grown in fresh DMEM-F12 complete + 15% FBS until 90% confluence. The fibroblasts were further expanded into three wells of a six-well plate and then trypsinized, collected, centrifuged, resuspended into 3 mL of complete media, passed through a 70 μm cell strainer (catalog no. 15-1070; Biologix), and finally plated into a 100 mm plate. For tissue harvest, lung were perfused through the right ventricle with 1× PBS, and then the trachea was cannulated with an 18-gauge cannula (catalog no. NC0553837; Fisher Scientific) and tied with a silk suture. The cannula was connected to a reservoir containing 10% buffered formalin (catalog no. S-182-1GL; Poli-Scientific), and the lungs were fixed in situ under 25 cm pressure for 15–20 min. After that, lungs were collected, fixed overnight in 10% buffered formalin, moved to 70% ethanol, and then dehydrated in increasing concentrations of ethanol. Lungs were embedded in paraffin, following standard procedures, sectioned at 5-μm thickness, and stained with hematoxylin and eosin (H&E) or Masson’s. In addition to these stains, lung sections were also stained with the Verhoeff-Van Gieson (VVG) staining protocol for elastic and collagen fibers, as described (http://www.ihcworld.com/_protocols/special_stains/vvg.htm). With these procedures, elastic fibers are stained blue-black while collagen fibers are stained red. The fixed lung volume was also measured: the lungs were harvested and treated as above except, after overnight formalin, the heart and other nonlung tissues were removed, and the fixed lung volume was measured by Archimedes’ principle of water displacement (32). To calculate the wet-to-dry lung weight (W/D) ratio, whole lungs were harvested from n = 6 male mice that were used in the forced oscillation measurements. The wet lung weight was assessed right after excision, whereas the dry lung weight was measured after 3–5 days of drying in an oven at 60°C. The W/D ratio was then calculated.
Lung Morphometry
The mean linear intercept was calculated to evaluate changes in lung morphometry. Images from at least 10 histological fields per mouse lung section (representative of distal airways) were captured at ×20 magnification using a Nikon microscope (Eclipse E400). The ImageJ software plug-in grid analysis was used to superimpose a grid over each image of size equal to 745.28 × 558.96 μm with 8 horizontal lines and 10 vertical lines. The number of times the grid lines intersected the alveolar wall was counted on each image. The equation used was Lm = [N horizontally + N vertically) × L]/I, where N is the number of times the transverse were placed on the tissue, L is length of the transverses, and I is the sum of all intercepts from each field (15).
Western Blotting
Murine whole lung tissues or primary lung fibroblasts in culture were lysed into RIPA buffer (50 mM Tris·HCl, pH 7.5, 150 mM NaCl, 0.1% SDS, 1 mM EDTA, 0.5% sodium deoxycholate, and 1% Triton X‐100) containing a cocktail of protease inhibitors, including EDTA (cAMRESCO, Solon, OH). The same procedure was used to generate a tissue lysate from human precision-cut lung slices obtained from an anonymous donor (41-yr-old female) through the Arkansas Organ Recovery Agency and processed as described (7). Lysates were centrifuged (15,000 g), and supernatants were collected and quantified using the Bio‐Rad protein assay dye reagent (catalog no. 5000006; Bio‐Rad, Hercules, CA). Proteins were separated by 10% SDS-PAGE according to standard techniques, transferred to a nitrocellulose membrane, and blocked for 30 min in 5% milk in TBS-T. Membranes were incubated with primary antibody diluted in TBS-T overnight at 4°C, followed by washing in TBS-T. Primary antibodies were Crtap (1:1,000; see Ref. 5) and β-actin (catalog no. A00702; GenScript, Piscataway, NJ). Secondary antibodies were goat anti‐rabbit (IRDye 800CW) or anti‐mouse (IRDye 680LT, catalog nos. 925–32211 and 925–68020, 1:20,000; LICOR Biosciences), and specimens were incubated for 1 h at room temperature. Membranes were scanned, and densitometry was performed using a LICOR Odyssey instrument with Image Studio version 5.2 software.
Collagen Extraction From Cell Culture Medium and Protein Mass Spectrometry
Media from 100 mm cell culture plates containing confluent lung fibroblasts were collected every other day in complete DMEM-F12 with 1% FBS plus ascorbic acid (100 μg/mL) to stimulate collagen production. Successive collections were stored frozen at −80°C. Medium aliquots were thawed on ice, transferred to a 15 mL tube and centrifuged at 0.5 g for 5 min to eliminate dead cells. Next, protease inhibitor (1×) was added (catalog no.1860932; Thermo Fisher), mixed and 25% vol/vol of saturated ammonium sulfate added, and put in rotation at 4°C O/N. The next day the samples were centrifuged at 25,000 rpm at 4°C for 90 min. Protein pellets were then resuspended with 1 mL of pepsin (0.1 mg/mL in 0.5 M acetic acid) and placed in rotation at 4°C O/N. Next, NaCl dissolved in acetic acid was added to a final concentration 0.9 M, and samples were rotated O/N at 4°C for the precipitation of type I collagen. The samples were then spun at 14,000 rpm at 4°C for 1 h, the pellets were washed with 400 µL of cold 70% ethanol, centrifuged at 14,000 rpm at 4°C for 20 min, and resuspended in 0.1 M Na2CO3, 0.5 M NaCl, pH 9.3. Collagen preps were loaded on a 5% gel urea-SDS in nonreducing conditions, run for ~3 h at 80 volts, and then stained for 1 h with Coomassie blue.
Portions of the Coomassie blue-stained gel were excised, destained in 50% methanol and 100 mM ammonium bicarbonate, followed by reduction in 10 mM Tris(2-carboxyethyl)phosphine and alkylation in 50 mM iodoacetamide. Gel slices were then dehydrated in acetonitrile, followed by addition of 100 ng porcine trypsin (Promega) in 100 mM ammonium bicarbonate and incubation at 37°C for ~14 h. Peptide products were then acidified in 0.1% formic acid. Tryptic peptides were analyzed by nanoflow LC-MS/MS with a Thermo Orbitrap Fusion mass spectrometer equipped with a Waters nanoACQUITY LC system as described previously (26). A MaxQuant (version 1.6.2.10; Max Planck Institute) database search was used to identify proteins and hydroxyproline modifications. The mass spectrometry results were searched against the UniprotKB database restricted to Mus musculus (85,390 entries; December 2018) with a parent ion tolerance of 3 ppm, a fragment ion tolerance of 0.5 Da, fixed modifications including carbamidomethyl on C, and variable modifications including hydroxyproline, deamidation on NQ, oxidation on M, and acetyl on the protein N-term. Protein identifications were accepted if they could be established with less than 1% false discovery and contained at least two identified peptides. Protein probabilities were assigned by the Protein Prophet algorithm (42). Proteins were normalized using the intensity-based absolute quantification (iBAQ) algorithm in MaxQuant. Hydroxyproline peptides were filtered by requiring the MaxQuant localization probability of modification detection to be greater than 75%. Next, hydroxyproline peptides with missing values were filtered out, and then the data were log2 transformed before analysis with Linear Models for Microarray Data (limma; see Ref. 46). To test differential expression between the immune-precipitated sample and the control, we used the limma R package to fit the hydroxyproline peptide intensity data to a linear model and then applied empirical Bayes statistics using the eBayes function to calculate moderated t statistics. Log2-fold changes were generated by subtracting a peptide’s intensity value in the immune-precipitated sample from its value in our negative control sample. Peptides with a fold change >2 and an FDR adjusted P value <0.05 were considered significantly enriched.
Whole Body Unrestrained Plethysmography
CrtapKO and Het/WT littermates (3- to 5-mo-old males, n = 12 for each genotype) were studied with whole body plethysmography (Fine Pointe; DSI Electronics) to measure respiratory parameters in conscious unrestrained subjects. Before each experiment, the four measuring chambers were calibrated according to the manufacturer’s instructions. For 5 days, mice were given the opportunity to acclimate to the testing by individually housing them inside the measuring chambers for at least 10 min every day. In the second week, mice were again individually housed inside the chambers, and their respiratory parameters were recorded during quiet wakefulness for 10 min/day, and similarly for the next 3 days. The values recorded over the 3–4 days were averaged for each single mouse and then averaged based on genotype. From the box flow signal, the following parameters were derived: inspiratory time (Ti); expiratory time (Te); tidal volume (VT); minute ventilation (V̇e); respiratory rate (F); total cycle time (Ttot), the Ti/Te ratio, Ti/Ttot ratio, and flow (V). Volume measurements were normalized by dividing by body weight.
Respiratory System Measurements Using the Flexivent Small Animal Ventilator
CrtapKO and Het/WT littermates (3-mo-old males, n = 8–10 for each genotype) were anesthetized with a mixture of ketamine (100 mg/kg ip) and xylazine hydrochloride (10 mg/kg ip). The trachea was exposed and freed from the surrounding tissues, and a suture silk passed underneath it. A small incision between the first and second tracheal ring was done, and the mice were cannulated with a bevel metal needle [18 gauge, 0.5 in. long, with a typical resistance of <0.2 cmH2O·s/mL; Scientific Respiratory Equipment Inc. (SCIREQ), Montreal, Quebec, Canada]. The cannula was tied with a suture silk, and the mice were connected to a small animal FlexiVent FX ventilator (SCIREQ; Montreal, Quebec, Canada) that was configured with an F2 module without nebulizer and operated by FlexiWare software version 8.0.4. Mechanical ventilation was set at 150 breath/min and a VT of 10 mL/kg and a positive end-expiratory pressure (PEEP) of 3 cmH2O. To block spontaneous respiratory effort, succinylcholine (7.5 mg/kg ip) was administered, and, preceding the mechanical perturbations, mice were ventilated for ~3 min to stabilize the subject and to allow the drug to reach its effect (47). During this time, deep inflations, 30 s apart, were performed.
The following perturbation sequence was applied:
Deep inflation.
Starting at FRC, airway opening pressure is increased in rampwise fashion to 30 cmH2O over 3 s and held at 30 cmH2O for 3 additional seconds. This maneuver serves to standardize volume history and recruit closed lung areas. The delivered volume, after correction for gas compression, represents inspiratory capacity.
Snapshot-150.
Snapshot-150 is a single-frequency forced oscillation technique (FOT) perturbation, delivering a 2.5-Hz sinusoidal oscillation matching VT (10 mL/kg) over 1.25 s. Analysis is based on a single compartment model yielding overall respiratory system resistance (Rrs), elastance (Ers), and compliance (Crs).
Quick prime-3.
Quick prime-3 is a complex, broadband forced-oscillation volume-driven perturbation that delivers a volume slightly smaller than tidal volume over an extended range of input frequencies between 1and 20.5 Hz. The measured impedance spectra is fitted by flexiWare software to the Constant Phase Model (24) to derive Newtonian resistance (Rn), tissue damping (G), tissue elasticity (H), and hysteresivity (G/H or “η”).
Pressure-volume curve.
We performed a pressure-driven maneuver during which the inflation typically starts at the set PEEP value, and then the subject’s lungs are inflated in a stepwise manner to a pressure of 30 cmH2O, after which the lungs are deflated in a stepwise manner back to the initial pressure. Volume and pressure signals are recorded at the end of each plateau period, and PV curves are automatically plotted in the software. The deflation arm of the curve is then fit with the exponential function described by Salazar and Knowles (48) from which parameters such as static compliance (Cst), A (an estimate of inspiratory capacity), k (shape constant that describes the curvature of the deflation arm), and area (representing the area between the inflation and deflation curves) can be obtained.
The above sequence was repeated two times at 1-min intervals for a total of three independent measurements per maneuver. Parameters from each maneuver were then averaged.
Lung volumes.
Lung volumes were measured from full-range PV curves using the method described by Robichaud et. al. (47). At the end of each subject’s measurements, a final maneuver was performed that starts with an inflation from zero volume. This is achieved by ventilating the subject with 100% O2 for 5 min after which the ventilation is stopped and the inspiratory and respiratory valves are closed for another 5 min while the alveolar gases are absorbed into the blood, effectively degassing the lungs (terminal procedure). After the degassing procedure, the piston is programmed to push air into the subject’s lung in a continuous manner and at a constant rate of 5 mL/min until the set pressure of 35 cmH2O is reached. Next, the piston pulls back to deflate the lungs until a pressure of −10 cmH2O is reached. This inflation/deflation sequence is repeated two more times without interruption for quality control. A full-range P-V curve is generated, and parameters such as total lung capacity (TLC), RV, C, and FRC estimate are automatically calculated.
Volume measurements from the deep inflation and P-V protocols are shown unadjusted and after normalization by dividing by body weight. All mechanical measurements were obtained with an intact chest wall. All measurements with a COD (coefficient of quality control determination) >90 were accepted and used for data analysis by averaging per subject and experimental group (25).
Statistical Analysis
All measured parameters are presented as means ± SD and were analyzed with the Student’s t test using a two-tailed distribution and two-sample equal variance as appropriate. P values <0.05 were considered statistically significant and reported as such. Graphs were plotted using GraphPad Prism 8 (GraphPad Software, San Diego, CA).
RESULTS
Crtap Expression in the Postnatal Lung
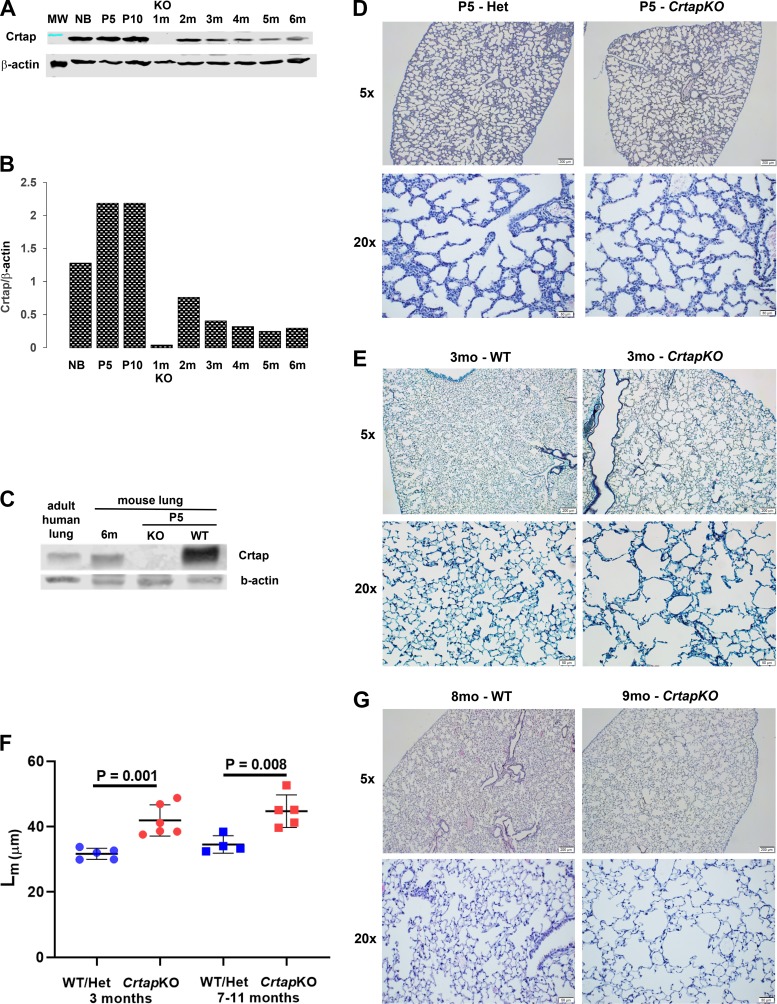
Using immunofluorescence, we previously showed that Crtap is expressed in the murine lung at postnatal day 10 (P10) (5). To confirm this and to assess Crtap expression levels during postnatal development and growth, we performed Western blot analysis of whole lung lysates prepared from WT mice at different ages and using a rabbit polyclonal antibody that recognizes the full-length Crtap protein (Fig. 1A). β-Actin was used to normalize the results, and bands in the gel were quantified with densitometry (Fig. 1B). Beginning at P0 [newborn (NB)], Crtap expression levels rose until P5 (5-day-old stage) and remained steady until P10. This increase appears to correlate with the lung alveolar phase during which the alveolar number and formation of the alveolar septa increase dramatically (Fig. 1B). At 2 mo of age, considered a late alveolar maturation stage in the murine model (9), the Crtap protein levels decrease significantly and seem to reach a stable, lower plateau at 6 mo. The expression of the protein was undetectable in the lung extract from CrtapKO mice, as expected. Furthermore, a Western blot that included tissue lysate from an adult human lung showed that the CRTAP protein is also expressed in the human organ (Fig. 1C).
Fig. 1.
Crtap expression and postnatal lung histology. A: Western blot analysis of wild-type (WT) whole lung lysates at different ages probed with a rabbit polyclonal antibody against CRTAP. A lysate of a 1-mo-old CrtapKO [knockout (KO)] lung was also included as the negative control. B: densitometry of the band products shown in A expressed as a ratio between Crtap and β-actin expression levels. NB, newborn; P5, 5 days old; P10, 10 days old; m, age in months. Results are representative from n = 1 sample/age and three technical replicates. C: Western blot showing CRTAP expression in adult human lung tissue. D, E, and G: histological sections of WT and CrtapKO lungs collected at P5, 3 mo, and 8–9 mo of age stained with hematoxylin and eosin (H&E) or Masson’s. F: quantification of the lung parenchyma defect at 3 mo and at 7–11 mo of age (n = 5 or 6 and n = 4 or 5 mice/genotype, respectively; 10 fields analyzed/mouse at ×20 magnification; Student’s t test) using the mean linear intercept (Lm) method.
Loss of Crtap Causes Primary Lung Defects
While the loss of Crtap protein causes a severe osteochondrodysplasia in mice and mimics a recessive form of OI (also described as OI type VII), we and others had shown that CrtapKO mice also have a primary defect in the lung (5, 22). Here, to further evaluate morphological changes of the lung architecture due to loss of Crtap expression and their age of onset, tissue histology was performed on lung sections from CrtapKO and WT mice at P5, 3 mo, and 7–11 mo of age. Sections were stained with hematoxylin-eosin and/or Masson’s trichrome. At P5 there appears to be no major changes in the lung parenchyma of the CrtapKO mice (Fig. 1D); however, at 3 mo of age, there are clear and dramatic differences between CrtapKO and WT mice, consisting of enlargement of the acinar airspace and frequent loss of alveolar septa (Fig. 1E). These defects were quantified using the mean linear intercept measurement and showed a statistically significant difference (Fig. 1F). Similar changes persisted in older mice (7–11 mo old; Fig. 1, F and G).
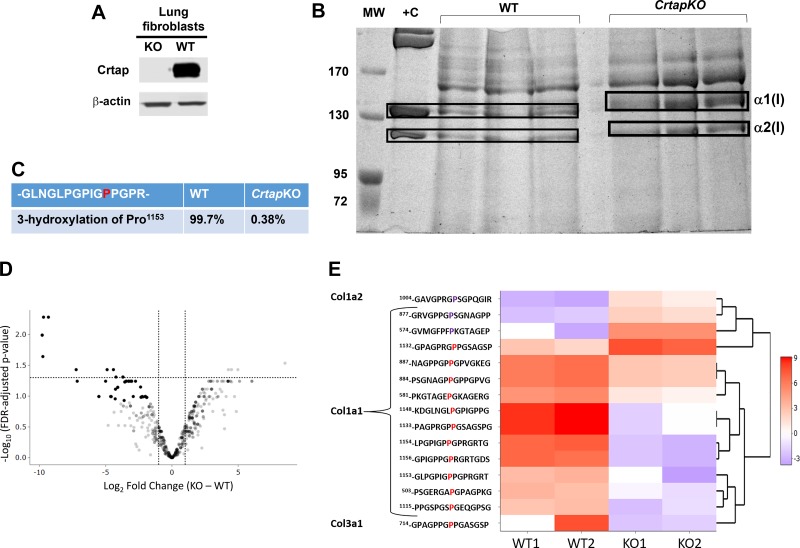
Lung Fibroblasts Produce Defective Type I Collagen in CrtapKO Mice
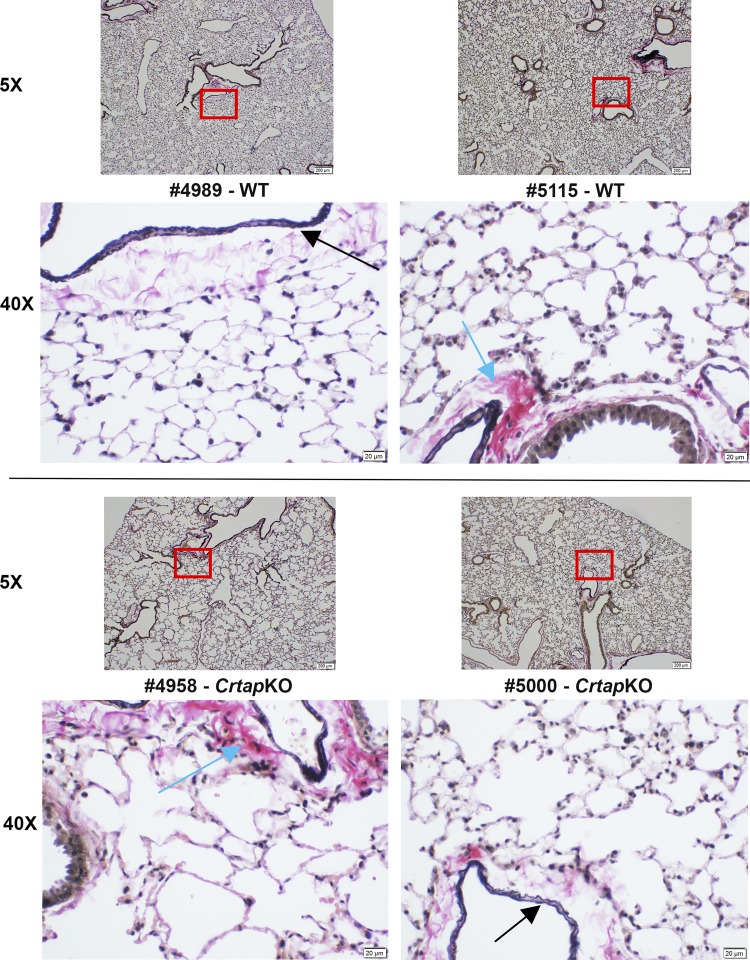
Lung fibroblasts are known to synthesize both collagen and elastin, which are responsible for the tensile strength and the elastic recoil of the lung, respectively (29, 30, 56, 63). The appearance, distribution, and integrity of both collagen and elastin fibers were then assessed with the Verhoeff-Van Gieson stain. While collagen and elastin fibers were clearly visible around cross sections of bronchioles and blood vessels, they were far less distinguishable, even at high magnification, at the alveolar wall level in either genotype (Fig. 2). No major qualitative differences could be observed between the two genotypes. Because Crtap is important for the posttranslational modification of fibrillar collagens and lung fibroblasts in the interstitial space of the alveolar walls express type I collagen, these cells were analyzed more in detail. Primary lung fibroblast cultures were obtained from P5 lungs and briefly expanded in vitro. A Western blot on primary lung fibroblast lysates showed that Crtap is indeed highly expressed in these cells but was completely lost in lung fibroblasts from CrtapKO mice (Fig. 3A). Next, the primary lung fibroblasts were stimulated with ascorbic acid, and culture media were collected one time daily every other day for 1 wk. The goal was to analyze type I collagen, which is normally expressed and secreted by the lung fibroblasts in the extracellular matrix of the alveolar septal wall. While type I collagen alterations, i.e., loss of 3Hyp and overmodification, were demonstrated in bone and skin of CrtapKO mice, it is unknown if lung collagen is also altered in this or other models of OI, especially since collagen PTMs can be tissue-specific. Collagen was extracted from the culture media, run on a 5% SDS-urea-PAGE, and stained with Coomassie blue (Fig. 3B). The gel showed a characteristic delay in the migration of α1(I) and α2(I) chains extracted from CrtapKO compared with WT/Het cells, indicating a delay in collagen folding and the consequent overmodification of the α-chains (Fig. 3B). Mass spectrometry analysis of the gel-excised type I collagen bands confirmed that Pro1153 (corresponding to Pro986 in the triple-helical collagen domain) was modified into 3Hyp (>99%) in WT/Het cells but lacked such modification in CrtapKO lung fibroblasts (Fig. 3C). Moreover, a survey of the mass-spectrometry data indicated that additional proline residues in type I collagen were differentially modified in CrtapKO compared with WT control fibroblasts, and several of these changes reached statistical significance (limma FDR P value < 0.05) as shown in Fig. 3, D and E. Several prolines were underhydroxylated although a few were overhydroxylated in type I collagen extracted from CrtapKO fibroblasts, as shown in the heat map, which depicts a hierarchical cluster of the scaled normalized peptide intensities and their modified peptide sequence (Fig. 3E). The flanking sequence of the modified amino acid and the position within the protein sequence are shown. The flanking sequence is the modified proline plus/minus seven amino acids. The peptide –GLPGPIGPPGPRGRT contains several prolines that are modified, and the peptides cluster together in the heatmap, indicating similarity of expression.
Fig. 2.
Histochemical staining of elastin and collagen fibers in wild-type (WT) and Crtap knockout (KO) lung sections. Representative images of WT (top) and CrtapKO (bottom) lung sections at 3 mo of age, stained with the Verhoeff-Van Gieson protocol. Elastic fibers are stained blue-black (black arrows) while collagen fibers are stained in red (light blue arrows). Images at ×40 are magnifications corresponding to the red boxed areas.
Fig. 3.
Collagen mass spectrometry from wild-type (WT) and Crtap knockout (KO) lung fibroblasts. A: Western blot of primary lung fibroblast lysates from CrtapKO and WT mice and probed with antibody against Crtap. B: electrophoretic migration of collagen extracted from media of WT and CrtapKO lung primary fibroblasts (n = 3). The gel was stained with Coomassie blue and shows electrophoretic migration delay of α1(I) and α2(I) chains in CrtapKO samples, characteristic of collagen overmodification. +C, positive control, is collagen extracted from rat tail (1 µg/µL). C–E: mass-spectrometry results showing loss of type I collagen 3Hyp at Pro1153 (previously referred to as Pro986 for processed Col1a1) in CrtapKO versus WT fibroblasts (C). D: a volcano plot showing the Linear Models for Microarray Data –log10 FDR adjusted P values and log2-fold changes for all peptides with a 3Hyp modification on prolines that were identified by mass spectrometry. E: a hierarchical cluster heat map displaying the scaled normalized peptide intensity values for each sample and significantly modified peptide. Peptides were considered significant with a fold change >2 and an FDR adjusted P value <0.05. These peptides are also visualized on the volcano plot. The sequence contains the position of the modified amino acid within the protein ± 7 amino acids. The proline in red and purple indicates the modified proline that is either over- or undermodified in WT versus CrtapKO. The numbering of amino acids starts from the first amino acid in the protein sequence.
Whole Body Plethysmography
Whole body plethysmography was performed to assess if the lung collagen and parenchyma alterations in CrtapKO mice would result in potential modifications of their breathing pattern at baseline. Because CrtapKO mice are ~20% smaller in body weight compared with their WT/Het littermates, the volume-dependent parameters are reported both as absolute values and normalized to the body weight. To determine the acceptability of normalizing volume-related measurements to body weight, the relationship between lung fixed volume and body weight (μL/g) was compared in a separate group of males. Lung fixed volume per unit body weight was 23.9 ± 3.7 μL/g in WT/Het subjects (n = 5) compared with 23 ± 2.3 μL/g (n = 6) in CrtapKO subjects [P = 0.6359, NS) (Supplemental Fig. S1A; see https://doi.org/10.6084/m9.figshare.11591841). Moreover, in an additional group of male mice (3 mo old), the wet and dry lung weights were recorded, and both body weight-to-wet lung weight and wet lung weight-to-dry lung weight ratios showed no significant differences between genotypes, indicating absence of pulmonary edema (Supplemental Fig. S1B; see https://doi.org/10.6084/m9.figshare.11591841). The plethysmography measurements from both male (n = 12) and female (n = 6) mice are shown combined in Table 1, given that there were no significant differences between sexes other than in a few volumetric measurements due to differences in body weight (Supplemental Table S2; see https://doi.org/10.6084/m9.figshare.11591832). There were no significant changes in inspiratory time (Ti) or total cycle time (Ttot) in CrtapKO compared with WT/Het mice. However, the expiratory time (Te) was decreased (P = 0.04), and the inspiratory-to-expiratory time ratio (Ti/Te) was significantly increased in CrtapKO mice (P = 0.002). The Ti/Ttot (inspiratory duty cycle) was also significantly increased (WT = 27.56% versus CrtapKO = 29.56%, P = 0.002; Table 1). The absolute values of tidal volume (VT), minute volume (V̇e), and flow rate (VT/Ti) were significantly lower in the CrtapKO group compared with WT/Het, but, when normalized to body weight, none of them retained statistical significance. The plethysmography measurements are also analyzed by sex and presented separately in Table 2. A small cohort of older mice (females only, n = 3) was also tested (Supplemental Table S1; see https://doi.org/10.6084/m9.figshare.11591817) and generally confirmed the findings shown in Table 1.
Table 1.
Results from the plethysmography measurements combining male and female data
| WT/Het | CrtapKO | t Test (P Value) | |
|---|---|---|---|
| Age 3–5 mo, n | 18 | 18 | |
| Weight, g | 24.43 ± 3.5 | 20.81 ± 2.5 | 0.001 |
| F, breaths/min | 356.13 ± 35.9 | 354.47 ± 38.8 | 0.89 |
| Ti, s | 0.058 ± 0.0056 | 0.059 ± 0.0072 | 0.64 |
| Te, s | 0.157 ± 0.0255 | 0.141 ± 0.0187 | 0.04 |
| Ttot, s | 0.215 ± 0.0299 | 0.2002 ± 0.0242 | 0.11 |
| Ti/Te | 0.372 ± 0.042 | 0.417 ± 0.039 | 0.002 |
| Ti/Ttot | 0.27 ± 0.0222 | 0.29 ± 0.0195 | 0.002 |
| Ti/Ttot % | 27.08 | 29.4 | |
| VT, mL | 0.28 ± 0.0294 | 0.23 ± 0.0264 | 0.000002 |
| VT/g, mL/g | 0.012 ± 0.002 | 0.011 ± 0.0016 | 0.24 |
| V̇e, mL/min | 100.94 ± 18.27 | 80.65 ± 15.02 | 0.0009 |
| V̇e/g, mL·min−1·g−1 | 4.21 ± 0.955 | 3.92 ± 0.791 | 0.33 |
| VT/Ti, mL/min | 294.29 ± 49.72 | 235.19 ± 41.03 | 0.0004 |
| VT/Ti/g, mL·min−1·g−1 | 12.31 ± 2.81 | 11.46 ± 2.32 | 0.32 |
Values are means ± SD. WT, wild type; KO, knockout; F, respiratory rate; Ti, inspiratory time; Te, expiratory time; Ttot, total cycle time; VT, tidal volume; V̇e, minute ventilation. Values in bold are significant (P < 0.05; Student’s t test).
Table 2.
Results from the plethysmography measurements: data from male and female mice are shown separately
| Males |
Females |
|||||
|---|---|---|---|---|---|---|
| WT/Het | CrtapKO | t Test (P Value) | WT/Het | CrtapKO | t Test (P Value) | |
| Age 3–5 mo, n | 12 | 12 | 6 | 6 | ||
| Weight, g | 25.96 ± 2.87 | 22.21 ± 1.16 | 0.0003 | 21.36 ± 2.57 | 18 ± 2.02 | 0.03 |
| F, breaths/min | 367.2 ± 35.52 | 351.16 ± 47.16 | 0.35 | 333.97 ± 26.96 | 361.08 ± 12.12 | 0.048 |
| Ti, s | 0.057 ± 0.006 | 0.06 ± 0.008 | 0.32 | 0.06 ± 0.005 | 0.057 ± 0.004 | 0.32 |
| Te, s | 0.15 ± 0.024 | 0.143 ± 0.022 | 0.42 | 0.171 ± 0.025 | 0.139 ± 0.008 | 0.014 |
| Ttot, s | 0.207 ± 0.029 | 0.202 ± 0.029 | 0.69 | 0.23 ± 0.028 | 0.196 ± 0.008 | 0.015 |
| Ti/Te | 0.381 ± 0.035 | 0.421 ± 0.041 | 0.017 | 0.355 ± 0.052 | 0.41 ± 0.037 | 0.061 |
| Ti/Ttot | 0.276 ± 0.018 | 0.296 ± 0.02 | 0.018 | 0.261 ± 0.028 | 0.291 ± 0.019 | 0.057 |
| Ti/Ttot % | 27.56 | 29.56 | 26.1 | 29.07 | ||
| VT, mL | 0.287 ± 0.028 | 0.227 ± 0.024 | 0.00001 | 0.265 ± 0.027 | 0.226 ± 0.031 | 0.044 |
| VT/g, mL/g | 0.011 ± 0.002 | 0.010 ± 0.001 | 0.17 | 0.012 ± 0.001 | 0.012 ± 0.0008 | 0.84 |
| V̇e, mL/min | 107.28 ± 19.2 | 80.31 ± 17.54 | 0.0016 | 88.23 ± 5.72 | 81.33 ± 9.42 | 0.15 |
| V̇e/g, mL·min−1·g−1 | 4.23 ± 1.15 | 3.621 ± 0.8 | 0.14 | 4.167 ± 0.432 | 4.522 ± 0.286 | 0.12 |
| VT/Ti, mL/min | 308.03 ± 55.91 | 233.62 ± 48.02 | 0.002 | 266.79 ± 12.84 | 238.34 ± 25.13 | 0.033 |
| VT/Ti/g, mL·min−1·g−1 | 12.15 ± 3.36 | 10.54 ± 2.22 | 0.18 | 12.85 ± 1.37 | 13.35 ± 1.12 | 0.5 |
Values are means ± SD. WT, wild type; KO, knockout; F, respiratory rate; Ti, inspiratory time; Te, expiratory time; Ttot, total cycle time; VT, tidal volume; V̇e, minute ventilation. Values in bold are significant (P < 0.05; Student’s t test).
Respiratory Mechanics Measurements Using the Forced Oscillation Technique and Pressure-Volume Curves
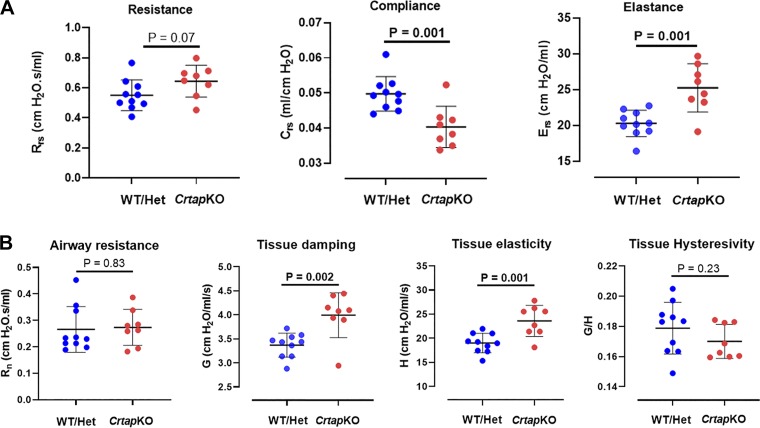
To further study the effects of the respiratory changes caused by the collagen defect on the respiratory mechanical properties and function, invasive studies were done using the forced oscillation technique (FOT) in closed-chest configuration in adult male mice (3 mo old, n = 8–10/genotype). Respiratory system elastance and compliance were highly significantly different (increased and decreased, respectively) in the CrtapKO mice compared with WT/Het controls (P = 0.001; Fig. 4A). Respiratory system resistance (Rrs) showed a tendency to increase in CrtapKO mice compared with control but did not achieve statistical significance (P = 0.07; Fig. 4A). Airways resistance (Rn) and tissue hysteresivity (G/H) were not different, but tissue damping (G) and tissue elasticity (H) were significantly higher (P = 0.002 and 0.001, respectively) in CrtapKO mice compared with WT/Het controls (Fig. 4B).
Fig. 4.
Results derived from the Snapshot 150 and the Quick prime-3 maneuvers using the forced oscillation technique (FOT). A: respiratory system elastance (Ers) and compliance (Crs) were significantly different (increased and decreased, respectively), while overall resistance (Rrs) of the respiratory system (i.e., including lungs, airways, and chest wall) did not reach statistical significance. B: tissue damping (G) and tissue elasticity (H) were both significantly increased; however, airways resistance (i) and tissue hysteresivity (G/H) were not changed in Crtap knockout (KO) compared with wild-type (WT)/Het mice. N = 8–10 each genotype. Student’s t test.
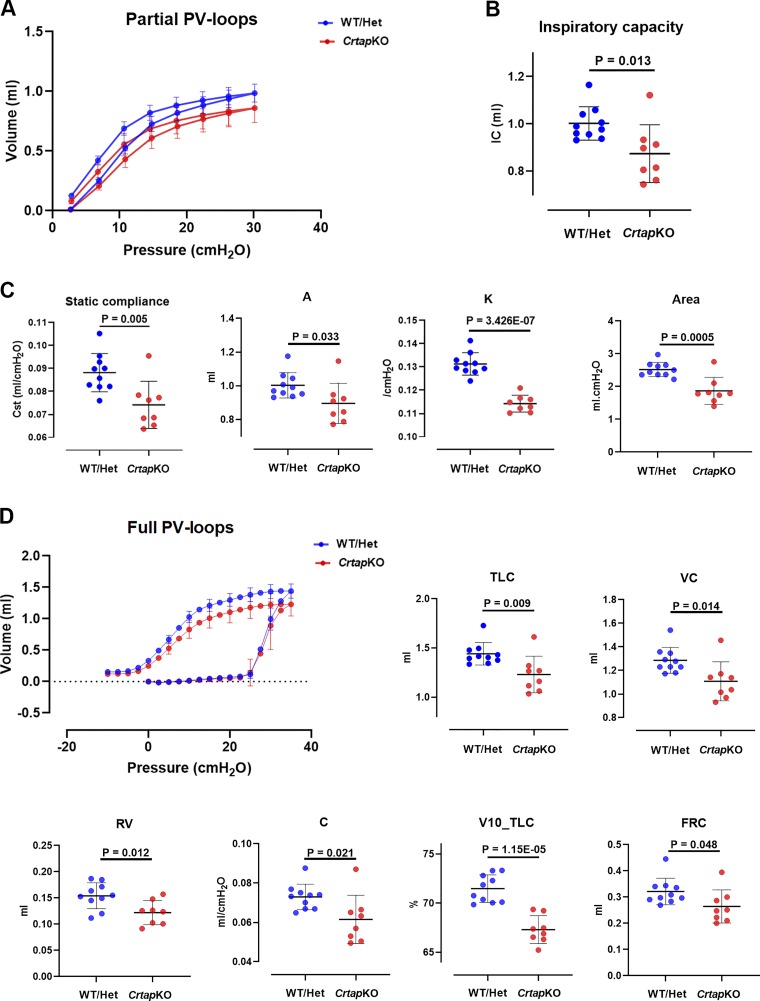
Partial pressure-volume (P-V) curves without adjustment for body weight showed a downward shift in CrtapKO mice (Fig. 5A). From these curves, static compliance (Cst), A (an estimate of inspiratory capacity), k (describes the curvature of the deflation arm), and area (the area between the inflation and deflation curves) were calculated, and all were consistently decreased in CrtapKO compared with control mice (Fig. 5C). The inspiratory capacity, calculated from the deep inflation maneuver, was also significantly reduced in CrtapKO mice (P = 0.013; Fig. 5B). After normalization of the partial P-V curves to body weight, in CrtapKO mice the portion of the curve <10 cmH2O shifted upward and was virtually identical to control (Supplemental Fig. S2A; see https://doi.org/10.6084/m9.figshare.11631918); static compliance became similar to control and did not retain statistical significance (Supplemental Fig. S2B; see https://doi.org/10.6084/m9.figshare.11631918). In contrast, the upward shift in the portion of the curve >10 cmH2O was larger such that inspiratory capacity was significantly greater in the CrtapKO mice [P = 0.002; Supplemental Fig. S2D (see https://doi.org/10.6084/m9.figshare.11631918).
Fig. 5.
Results derived from deep inflation and pressure-volume (P-V) maneuvers. A: partial P-V loops and the related parameters that were derived from these curves (C). Cst, static compliance; A, an estimate of inspiratory capacity; k, shape constant that describes the curvature of the deflation arm; area, area between the inflation and deflation curves. B: inspiratory capacity as measured with the deep inflation maneuvers. D: full-range P-V loops and the derived parameters, including total lung capacity (TLC), vital capacity (VC), residual volume (RV), compliance (C), volume at 10 cmH2O as percentage of TLC (V10_TLC), and an estimate of functional residual capacity (FRC). All parameters were significantly decreased in Crtap knockout (KO) compared with wild-type (WT)/Het mice. N = 8–10 each genotype. Student’s t test.
Nonnormalized full-range P-V curves showed a downward shift in the P-V curve of CrtapKO mice, and all derived variables, including TLC, vital capacity (VC), residual volume (RV), compliance (C), volume at 10 cmH2O as percent of TLC (V10_TLC), and functional residual capacity (FRC), were significantly reduced in CrtapKO compared with control mice (Fig. 5D). Following normalization to body weight, the CrtapKO P-V curve shifted upward such that the lower portion of the curve (<10 cmH2O pressure) became superimposed on control, and RV, FRC, and quasistatic compliance (measured from lower portion of the curve) became nonsignificant (NS) (Supplemental Fig. S2, C and E; see https://doi.org/10.6084/m9.figshare.11631918). In contrast, after normalization, the upper portion of the curve (>10 cmH2O pressure) was greater in CrtapKO mice than control, such that TLC, VC, and IC became significantly elevated versus control (Supplemental Fig. S2, C and E; see https://doi.org/10.6084/m9.figshare.11631918). V10_TLC, an index of the shape of the curve, remained significantly lower in the CrtapKO mice compared with control, even after normalization to body weight (Supplemental Fig. S2E; see https://doi.org/10.6084/m9.figshare.11631918).
DISCUSSION
Our understanding of the genetic and clinical heterogeneity of OI has improved significantly in recent years; however, there are extraskeletal manifestations of the disease, such as compromised respiratory function, that are still poorly understood but represent a leading cause of mortality (16). Traditionally, abnormal pulmonary function in OI was considered a consequence of multiple rib fractures and orthopedic complications of the spine, including scoliosis, kyphosis, and vertebral compressions that can lead to poor pulmonary ventilation and cause a progressive decrease in cardiorespiratory fitness (2, 17). Others, however, have suggested that type I collagen alterations could affect the development and function of the lung (50, 65). Indeed, despite differences in disease severity, all OI patients are at increased risk due to lung infections, with a progressive decline in lung function that can lead to respiratory failure and death (58). In this study, we hypothesized that type I collagen alterations in OI cause a primary defect in the lung and negatively impact respiratory function. Using the CrtapKO mouse model of recessive OI, we confirm postnatal primary defects in the lung parenchyma that are reminiscent of emphysematous changes. We also demonstrate that type I collagen in the lung is improperly posttranslationally modified, and these defects may contribute to the observed changes in the lung parenchyma. Finally, these alterations cause changes in the respiratory pattern of the mice and affect their respiratory mechanics, with alterations in tissue properties and pressure-volume relationships compared with controls.
The data from our Western blots clearly indicate that Crtap is expressed in the murine postnatal lung. There is a negative correlation between protein level and age: Crtap highest levels of expression are detected in the first 2–3 wk after birth, which coincide with the alveolar stage of the lung and correlate with a large increase in alveolar number and alveolar septa formation requiring synthesis of interstitial collagen (9). The expression of Crtap progressively decreases until ~2 mo of age, corresponding to the final maturation stage that occurs between P21 to P56 after birth (9, 62). The expression then stabilizes to a lower plateau after the third month of age and likely reflects the decreased need of Crtap and the prolyl 3-hydroxylation complex as lung organogenesis is complete, but lung growth and formation of alveoli continues, albeit to a lower degree, up to 9 mo of age (43). Importantly, Crtap is also expressed in the human lung, which suggests a preserved similar function. In humans, the alveolar stage of lung development begins in utero and continues postnatally for a few years (12, 62). We described earlier that the loss of Crtap causes increased acinar airspaces and thinning of the alveolar walls that were visible at P10 (5). In this study, we show that, at P5, the lung histology appears rather normal and, therefore, the onset of the morphological changes must take place between P5 and P10. By 3 mo of age, the lung parenchyma is significantly and diffusely altered, suggesting either a widespread loss of alveolar integrity or an impairment of lung development with failure to properly form alveoli. These morphological changes are seen in the adult lung (7–11 mo old) as well and thus constitute long-term tissue abnormalities that do not appear to worsen nor improve with time. We believe these data strongly support the hypothesis that a defect in collagen biosynthesis results in primary lung alterations. Type I and III collagen are the most abundant collagens in the lung, in a ratio of ~2:1 (30). They are secreted by fibroblasts and codistribute in the airways, blood vessels, and the interstitium (30, 63). The tensile strength of collagen is very important because it is the load-bearing constituent of the alveolar wall and protects it from failure due to mechanical stress (11, 56). It is possible that the collagen biochemical modifications observed in CrtapKO mice and the consequent potential alterations in cross-linking of the collagen fibrils in OI may significantly weaken the strength of the alveolar wall and its ability to resist the mechanical stress and strain that are typical of each breathing cycle. In addition, CrtapKO fibroblasts were shown by others to depose significantly less collagen in the ECM (60). Therefore, these data collectively suggest that collagen alterations in quantity, structure, and perhaps even geometry of distribution could cause early rupture of the alveolar wall or developmental dysplasia of the lung parenchyma with defective alveolarization. This would result in an increase of the acinar airspace as seen in CrtapKO mice and in two other OI mouse models, the Aga2 and Col1a1Jrt/+ (4, 58). Our data are consistent with previous findings on lung parenchyma structural changes induced by lathyrogenic compounds that affect collagen cross-linking (54).
The described effects are likely to alter lung function, and, to verify this hypothesis, we performed both noninvasive measurements with unrestrained whole body plethysmography and invasive studies of respiratory mechanics using the forced oscillation technique and pressure-volume maneuvers (14, 20, 38). Baseline measurements in unrestrained mice indicated clear differences in respiratory timing and pattern between controls and CrtapKO mice. However, none of the volume-related tidal breathing measurements (VT, V̇e, or VT/Ti) differed between controls and CrtapKO mice after adjusting for their lower body weight (Table 1 and Supplemental Fig. S1). Breathing pattern differences have also been reported in patients with OI; minute ventilation was reported to be similar to controls but with a pattern of increased frequency and rapid, shallow breathing pattern with smaller VT (33, 34).
FOT measurements (Snapshot-150 and Quick prime-3) indicated lower Crs and higher Ers in CrtapKO mice (Fig. 4A). Although there was a trend for Rrs to be greater in Crtap mice, it did not meet statistical significance (Fig. 4A). Newtonian resistance, which reflects mainly conducting airways, was not significantly different between controls and CrtapKO mice, suggesting that any increase in Rrs in CrtapKO mice would be due to differences in tissue and/or chest wall resistance rather than airways resistance. Tissue damping (G), which reflects tissue resistance, and tissue elasticity (H), which reflects tissue stiffness, were both significantly greater in CrtapKO mice without significant differences in hysteresivity (G/H; Fig. 4B).
Nonnormalized full-range and partial pressure-volume measurements all showed, in CrtapKO mice, smaller lung volumes, lower static compliance, and flattened shape of the expiratory arm of the P-V curve (Fig. 5). Smaller volumes could be explained in part by the smaller body size of the CrtapKO mice. However, the shape parameter k, derived from the partial P-V curve, is not size-dependent and was clearly lower in the CrtapKO group, consistent with reduced curvature characteristic of a restrictive pattern (Fig. 5A). In addition, V10_TLC from the full-range P-V curves, the volume at 10 cmH2O pressure as percent of TLC, also independent of body size, was clearly reduced in the CrtapKO group (even after normalization to body weight), indicating a flattening of the expiratory arm of the P-V curve as seen in disorders such as fibrosis (31).
Studies of respiratory mechanics in mice, if data are normalized at all, have normalized to body weight, lung weight, lung size, or lung volumes such as FRC or TLC (21, 27, 45). We elected not to normalize measures from FOT maneuvers, in part because these volume-driven perturbations (Snapshot-150 and Quick prime-3) are scaled to the subject’s tidal volume. Normalization of P-V data to lung volume (FRC or TLC) was considered. However, at any inflation pressure, lung volume depends on the elastic properties of the chest wall and lungs, both of which may be affected by collagen defects in the CrtapKO group. This would result in normalization to a variable of interest that may itself be altered by defective collagen in CrtapKO mice. Normalization to lung weight was not an option; although we report lung weight and fixed volume for a subgroup of control versus CrtapKO mice, we did not determine lung weight in all of the subjects in which FOT measures and P-V curves were performed. Finally, it was decided that normalization of deep inflation and P-V results to body weight was most appropriate, especially given that the relationship between lung weight and body weight was not significantly different between controls and CrtapKO mice (Supplemental Fig. S1). As noted above, several size-independent measures are suggestive of less-compliant lungs in the CrtapKO group, supported by results from the FOT measurements.
After normalizing P-V measures by expressing per gram of body weight, the expiratory arms of the P-V curves (both partial and full range) become nearly identical at pressures below 10 cmH2O. In contrast, at pressures above 10 cmH2O, the P-V curves did not simply become identical, as might be expected if body weight were the only factor accounting for control versus Crtap differences, and TLC, IC, and VC in the CrtapKO group became significantly greater compared with controls (Supplemental Fig. S2; see https://doi.org/10.6084/m9.figshare.11631918). This is similar to findings of Vanoirbeek et al. and others showing elevation of lung volumes in a mouse model of emphysema (61). However, in the CrtapKO mice after normalization to body weight, volumes at pressures <10 cmH2O collapsed on the same curve as controls, and compliance measured in the range of tidal breathing pressures (e.g., 3–8 cmH2O) was not different from control (NS) (Supplemental Fig. S2A). The normalized results suggest that, in the CrtapKO mice, the static compliance is significantly different at maximal inflation pressure but not at pressures required for tidal ventilation. This is consistent with the shape parameter k and V10_TLC, which were both lower in CrtapKO mice, even after normalization of P-V curves to body weight. Elastin has long been thought to be the major determinant of pressure-volume relationships at lower lung volumes while collagen has been viewed as dominant at high lung volumes (49). Thus, it is possible that CrtapKO mice have increased respiratory system compliance at high pressures (30–35 cmH2O) but not at the pressures associated with tidal ventilation (~3–8 cmH2O).
Although the structural changes in CrtapKO mice and the normalized P-V results are consistent with emphysema-like abnormalities, FOT measures in CrtapKO mice do not fully align with reported findings in rodent models of emphysema. Mouse or rat emphysema models using elastolytic injury to effect changes in lung structure typically show increased lung volumes, reduced elastance [Ers, tissue elasticity (H)], increased compliance (Crs), and elevation of hysteresivity (G/H) (14, 23, 59, 61). The increase in hysteresivity observed in elastase-emphysema models (14, 23, 61) was mainly due to a decrease in tissue elasticity (H), which differs from CrtapKO mice that showed an increase in both G and H and no difference in hysteresivity (G/H). In contrast, in a mouse model of protease-induced emphysema, tissue elastance (H) and tissue resistance (G) were not different from control at a time when marked emphysematous changes were present in lung tissue, indicating that respiratory mechanics may not show alterations in G or H in some murine models with pulmonary emphysema (3). Similarly, although cigarette smoke induced marked emphysematous changes in two strains of mice, open-chest measurements indicated that lung compliance was decreased or unchanged compared with nonexposed mice in a strain-dependent manner (19), again indicating that the respiratory mechanics associated with “emphysema” can vary widely depending on the mechanisms underlying the emphysematous changes. Murine models with emphysema induced by elastase, proteases, cigarette smoke, and other insults reflect acute short-term lung and airway injury, resulting in emphysematous changes that may differ considerably from structural lung abnormalities developing chronically over a lifetime due to abnormal collagen formation.
In summary, CrtapKO mice appear to exhibit a unique respiratory phenotype. Unrestrained plethysmography results, after normalization to body weight, indicate differences in breathing pattern/timing, but not volume within the tidal breathing range. Quasistatic partial and full-range P-V curves, after normalization to body weight, are similar in the tidal breathing range but show significant elevation of lung volumes at pressures greater than ~10 cmH2O, in strong contrast to elastase-induced emphysema models that show increased respiratory volumes across the entire pressure range (47, 61). Single-frequency and broadband FOT measurements are consistent with increased tissue damping and elasticity without changes in respiratory system or Newtonian resistance. Future studies from our laboratory will further address the issue of normalization to body size by studying weight-matched groups of CrtapKO and control mice within an age range when respiratory effects of collagen mutations are stable.
An important limitation of the current study derives from the embedding of the lungs in paraffin. Although processing of the lung samples was done identically in the two genotypes, there could be differences in tissue shrinkage that could lead to tissue deformations and thus impact our morphometric measurements (Lm), to a certain degree. Additional limitations with regard to the pulmonary function measurements should be noted. All of the FOT results reflect total respiratory system mechanics, which may be affected by the chest wall mechanics. CrtapKO mice exhibit reduced body size and kyphosis similar to PPIB knockout mice (13) and, due to abnormalities of bone and connective tissue, likely have higher chest wall compliance compared with controls. A previous study in normal BALB/c mice using closed versus open-chest measurements indicated that the chest wall contributed ~20% to G, ~6% to Rn, and did not affect H (53) while another study using C57BL/6 mice concluded that dynamic pulmonary mechanics in mice reflect only a minor contribution from the chest wall (28). However, a recent study on adult BALB/c mice reported that the chest wall may contribute ~40% to G and ~30% to H (55). There are no studies to date on the contribution of chest wall to respiratory mechanics in any model of OI. Therefore, the respiratory phenotype of the CrtapKO mice may in part reflect rib cage deformities, and further studies, including open- versus closed-chest approaches to separate chest wall and pulmonary impedances, will be needed to precisely define all components of pulmonary dysfunction in the CrtapKO and other OI models.
In conclusion, our data strongly indicate that type I collagen alterations in OI not only cause brittle bones but also a primary lung defect that affects the respiratory system and clearly needs future studies to be further characterized.
GRANTS
The research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under Award Number R03 HD-097559. Support also came from the University of Arkansas for Medical Sciences (UAMS) College of Medicine Research Scholar Pilot Grant Award in Child Health, UAMS institutional funds, National Institute of General Medical Sciences Grants P20 GM-125503 and P20 GM-121293 and National Center for Advancing Translational Sciences Grant U54 TR-001629.
DISCLAIMERS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
M.D., J.L.C., and R.M. conceived and designed research; M.D., M.E.H.-L., S.D.B., S.G.M., and R.C.K. performed experiments; M.D., S.D.B., S.G.M., J.L.C., and R.M. analyzed data; M.D., S.D.B., J.L.C., and R.M. interpreted results of experiments; M.D., S.D.B., and R.M. prepared figures; M.D., J.L.C., and R.M. drafted manuscript; M.D., M.E.H.-L., S.D.B., J.L.C., and R.M. edited and revised manuscript; M.D., M.E.H.-L., S.D.B., S.G.M., R.C.K., J.L.C., and R.M. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Dr. Roger Pechous (Department of Microbiology & Immunology, University of Arkansas for Medical Sciences) for allowing access and use to the Buxco whole body plethysmography instrument and Dr. Mark Lawrence (SCIREQ Inc., Montreal, Quebec, CA) for helpful discussions about the respiratory mechanics measurements.
Present address of M. E. Heard-Lipsmeyer: Div. of Developmental Nutrition, Dept. of Pediatrics, University of Arkansas for Medical Sciences, Little Rock, AR.
REFERENCES
- 1.Alapati D, Shaffer TH. Skeletal dysplasia: Respiratory management during infancy. Respir Med 131: 18–26, 2017. doi: 10.1016/j.rmed.2017.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alharbi SA. A systematic overview of osteogenesis imperfecta. Mol Biol 5: 1, 2016. doi: 10.4172/2168-9547.1000150. [DOI] [Google Scholar]
- 3.Anciães AM, Olivo CR, Prado CM, Kagohara KH, Pinto TS, Moriya HT, Mauad T, Martins MA, Lopes FD. Respiratory mechanics do not always mirror pulmonary histological changes in emphysema. Clinics (Sao Paulo) 66: 1797–1803, 2011. doi: 10.1590/s1807-59322011001000020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baglole CJ, Liang F, Traboulsi H, Rico de Souza A, Giordano C, Tauer JT, Rauch F, Petrof BJ. Pulmonary and diaphragmatic pathology in collagen type I α1 mutant mice with osteogenesis imperfecta. Pediatr Res 83: 1165–1171, 2018. doi: 10.1038/pr.2018.36. [DOI] [PubMed] [Google Scholar]
- 5.Baldridge D, Lennington J, Weis M, Homan EP, Jiang MM, Munivez E, Keene DR, Hogue WR, Pyott S, Byers PH, Krakow D, Cohn DH, Eyre DR, Lee B, Morello R. Generalized connective tissue disease in Crtap-/- mouse. PLoS One 5: e10560, 2010. doi: 10.1371/journal.pone.0010560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baldridge D, Schwarze U, Morello R, Lennington J, Bertin TK, Pace JM, Pepin MG, Weis M, Eyre DR, Walsh J, Lambert D, Green A, Robinson H, Michelson M, Houge G, Lindman C, Martin J, Ward J, Lemyre E, Mitchell JJ, Krakow D, Rimoin DL, Cohn DH, Byers PH, Lee B. CRTAP and LEPRE1 mutations in recessive osteogenesis imperfecta. Hum Mutat 29: 1435–1442, 2008. doi: 10.1002/humu.20799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerjee SK, Huckuntod SD, Mills SD, Kurten RC, Pechous RD. Modeling pneumonic plague in human precision-cut lung slices highlights a role for the plasminogen activator protease in facilitating type 3 secretion. Infect Immun 87: e00175-19, 2019. doi: 10.1128/IAI.00175-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnes AM, Chang W, Morello R, Cabral WA, Weis M, Eyre DR, Leikin S, Makareeva E, Kuznetsova N, Uveges TE, Ashok A, Flor AW, Mulvihill JJ, Wilson PL, Sundaram UT, Lee B, Marini JC. Deficiency of cartilage-associated protein in recessive lethal osteogenesis imperfecta. N Engl J Med 355: 2757–2764, 2006. doi: 10.1056/NEJMoa063804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beauchemin KJ, Wells JM, Kho AT, Philip VM, Kamir D, Kohane IS, Graber JH, Bult CJ. Temporal dynamics of the developing lung transcriptome in three common inbred strains of laboratory mice reveals multiple stages of postnatal alveolar development. PeerJ 4: e2318, 2016. doi: 10.7717/peerj.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besio R, Forlino A. Treatment options for osteogenesis imperfecta. Expert Opin Orphan Drugs 3: 165–181, 2015. doi: 10.1517/21678707.2015.1006197. [DOI] [Google Scholar]
- 11.Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 50: 1601805, 2017. doi: 10.1183/13993003.01805-2016. [DOI] [PubMed] [Google Scholar]
- 12.Chao CM, El Agha E, Tiozzo C, Minoo P, Bellusci S. A breath of fresh air on the mesenchyme: impact of impaired mesenchymal development on the pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne) 2: 27, 2015. doi: 10.3389/fmed.2015.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choi JW, Sutor SL, Lindquist L, Evans GL, Madden BJ, Bergen HR III, Hefferan TE, Yaszemski MJ, Bram RJ. Severe osteogenesis imperfecta in cyclophilin B-deficient mice. PLoS Genet 5: e1000750, 2009. doi: 10.1371/journal.pgen.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Devos FC, Maaske A, Robichaud A, Pollaris L, Seys S, Lopez CA, Verbeken E, Tenbusch M, Lories R, Nemery B, Hoet PH, Vanoirbeek JA. Forced expiration measurements in mouse models of obstructive and restrictive lung diseases. Respir Res 18: 123, 2017. doi: 10.1186/s12931-017-0610-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dunnill MS. Quantitative methods in the study of pulmonary pathology. Thorax 17: 320–328, 1962. doi: 10.1136/thx.17.4.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Folkestad L, Hald JD, Canudas-Romo V, Gram J, Hermann AP, Langdahl B, Abrahamsen B, Brixen K. Mortality and causes of death in patients with osteogenesis imperfecta: a register-based nationwide cohort study. J Bone Miner Res 31: 2159–2166, 2016. doi: 10.1002/jbmr.2895. [DOI] [PubMed] [Google Scholar]
- 17.Folkestad L, Hald JD, Gram J, Langdahl BL, Hermann AP, Diederichsen AC, Abrahamsen B, Brixen K. Cardiovascular disease in patients with osteogenesis imperfecta - a nationwide, register-based cohort study. Int J Cardiol 225: 250–257, 2016. doi: 10.1016/j.ijcard.2016.09.107. [DOI] [PubMed] [Google Scholar]
- 18.Forlino A, Marini JC. Osteogenesis imperfecta. Lancet 387: 1657–1671, 2016. doi: 10.1016/S0140-6736(15)00728-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foronjy RF, Mercer BA, Maxfield MW, Powell CA, D’Armiento J, Okada Y. Structural emphysema does not correlate with lung compliance: lessons from the mouse smoking model. Exp Lung Res 31: 547–562, 2005. doi: 10.1080/019021490951522. [DOI] [PubMed] [Google Scholar]
- 20.Glaab T, Taube C, Braun A, Mitzner W. Invasive and noninvasive methods for studying pulmonary function in mice. Respir Res 8: 63, 2007. doi: 10.1186/1465-9921-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gomes RF, Shen X, Ramchandani R, Tepper RS, Bates JH. Comparative respiratory system mechanics in rodents. J Appl Physiol (1985) 89: 908–916, 2000. doi: 10.1152/jappl.2000.89.3.908. [DOI] [PubMed] [Google Scholar]
- 22.Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, Bertin T, Munivez E, Chen Y, Dawson B, Ishikawa Y, Weis MA, Sampath TK, Ambrose C, Eyre D, Bächinger HP, Lee B. Excessive transforming growth factor-β signaling is a common mechanism in osteogenesis imperfecta. Nat Med 20: 670–675, 2014. doi: 10.1038/nm.3544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hantos Z, Adamicza A, Jánosi TZ, Szabari MV, Tolnai J, Suki B. Lung volumes and respiratory mechanics in elastase-induced emphysema in mice. J Appl Physiol (1985) 105: 1864–1872, 2008. doi: 10.1152/japplphysiol.90924.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hantos Z, Daróczy B, Suki B, Nagy S, Fredberg JJ. Input impedance and peripheral inhomogeneity of dog lungs. J Appl Physiol (1985) 72: 168–178, 1992. doi: 10.1152/jappl.1992.72.1.168. [DOI] [PubMed] [Google Scholar]
- 25.Hartney JM, Robichaud A. Assessment of airway hyperresponsiveness in mouse models of allergic lung disease using detailed measurements of respiratory mechanics. Methods Mol Biol 1032: 205–217, 2013. doi: 10.1007/978-1-62703-496-8_16. [DOI] [PubMed] [Google Scholar]
- 26.Heard ME, Besio R, Weis M, Rai J, Hudson DM, Dimori M, Zimmerman SM, Kamykowski JA, Hogue WR, Swain FL, Burdine MS, Mackintosh SG, Tackett AJ, Suva LJ, Eyre DR, Morello R. Sc65-null mice provide evidence for a novel endoplasmic reticulum complex regulating collagen lysyl hydroxylation. PLoS Genet 12: e1006002, 2016. doi: 10.1371/journal.pgen.1006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hirai T, Hosokawa M, Kawakami K, Takubo Y, Sakai N, Oku Y, Chin K, Ohi M, Higuchi K, Kuno K, Mishima M. Age-related changes in the static and dynamic mechanical properties of mouse lungs. Respir Physiol 102: 195–203, 1995. doi: 10.1016/0034-5687(95)00068-2. [DOI] [PubMed] [Google Scholar]
- 28.Ito S, Lutchen KR, Suki B. Effects of heterogeneities on the partitioning of airway and tissue properties in normal mice. J Appl Physiol (1985) 102: 859–869, 2007. doi: 10.1152/japplphysiol.00884.2006. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni T, O’Reilly P, Antony VB, Gaggar A, Thannickal VJ. Matrix remodeling in pulmonary fibrosis and emphysema. Am J Respir Cell Mol Biol 54: 751–760, 2016. doi: 10.1165/rcmb.2015-0166PS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Laurent GJ. Lung collagen: more than scaffolding. Thorax 41: 418–428, 1986. doi: 10.1136/thx.41.6.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Limjunyawong N, Fallica J, Horton MR, Mitzner W. Measurement of the pressure-volume curve in mouse lungs. J Vis Exp: 52376, 2015. doi: 10.3791/52376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Limjunyawong N, Mock J, Mitzner W. Instillation and fixation methods useful in mouse lung cancer research. J Vis Exp: e52964, 2015. doi: 10.3791/52964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LoMauro A, Fraschini P, Pochintesta S, Romei M, D’Angelo MG, Aliverti A. Ribcage deformity and the altered breathing pattern in children with osteogenesis imperfecta. Pediatr Pulmonol 53: 964–972, 2018. doi: 10.1002/ppul.24039. [DOI] [PubMed] [Google Scholar]
- 34.LoMauro A, Pochintesta S, Romei M, D’Angelo MG, Pedotti A, Turconi AC, Aliverti A. Rib cage deformities alter respiratory muscle action and chest wall function in patients with severe osteogenesis imperfecta. PLoS One 7: e35965, 2012. doi: 10.1371/journal.pone.0035965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marini JC, Blissett AR. New genes in bone development: what’s new in osteogenesis imperfecta. J Clin Endocrinol Metab 98: 3095–3103, 2013. doi: 10.1210/jc.2013-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marini JC, Forlino A, Bächinger HP, Bishop NJ, Byers PH, Paepe A, Fassier F, Fratzl-Zelman N, Kozloff KM, Krakow D, Montpetit K, Semler O. Osteogenesis imperfecta. Nat Rev Dis Primers 3: 17052, 2017. doi: 10.1038/nrdp.2017.52. [DOI] [PubMed] [Google Scholar]
- 37.McAllion SJ, Paterson CR. Causes of death in osteogenesis imperfecta. J Clin Pathol 49: 627–630, 1996. doi: 10.1136/jcp.49.8.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGovern TK, Robichaud A, Fereydoonzad L, Schuessler TF, Martin JG. Evaluation of respiratory system mechanics in mice using the forced oscillation technique. J Vis Exp: e50172, 2013. doi: 10.3791/50172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morello R, Bertin TK, Chen Y, Hicks J, Tonachini L, Monticone M, Castagnola P, Rauch F, Glorieux FH, Vranka J, Bächinger HP, Pace JM, Schwarze U, Byers PH, Weis M, Fernandes RJ, Eyre DR, Yao Z, Boyce BF, Lee B. CRTAP is required for prolyl 3- hydroxylation and mutations cause recessive osteogenesis imperfecta. Cell 127: 291–304, 2006. doi: 10.1016/j.cell.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 40.Morello R, Rauch F. Role of cartilage-associated protein in skeletal development. Curr Osteoporos Rep 8: 77–83, 2010. doi: 10.1007/s11914-010-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munns CF, Rauch F, Mier RJ, Glorieux FH. Respiratory distress with pamidronate treatment in infants with severe osteogenesis imperfecta. Bone 35: 231–234, 2004. doi: 10.1016/j.bone.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal Chem 75: 4646–4658, 2003. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 43.Pozarska A, Rodríguez-Castillo JA, Surate Solaligue DE, Ntokou A, Rath P, Mižíková I, Madurga A, Mayer K, Vadász I, Herold S, Ahlbrecht K, Seeger W, Morty RE. Stereological monitoring of mouse lung alveolarization from the early postnatal period to adulthood. Am J Physiol Lung Cell Mol Physiol 312: L882–L895, 2017. doi: 10.1152/ajplung.00492.2016. [DOI] [PubMed] [Google Scholar]
- 44.Rauch F, Glorieux FH. Osteogenesis imperfecta. Lancet 363: 1377–1385, 2004. doi: 10.1016/S0140-6736(04)16051-0. [DOI] [PubMed] [Google Scholar]
- 45.Reinhard C, Eder G, Fuchs H, Ziesenis A, Heyder J, Schulz H. Inbred strain variation in lung function. Mamm Genome 13: 429–437, 2002. doi: 10.1007/s00335-002-3005-6. [DOI] [PubMed] [Google Scholar]
- 46.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, Smyth GK. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43: e47, 2015. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robichaud A, Fereydoonzad L, Limjunyawong N, Rabold R, Allard B, Benedetti A, Martin JG, Mitzner W. Automated full-range pressure-volume curves in mice and rats. J Appl Physiol (1985) 123: 746–756, 2017. doi: 10.1152/japplphysiol.00856.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Salazar E, Knowles JH. An analysis of pressure-volume characteristics of the lungs. J Appl Physiol 19: 97–104, 1964. doi: 10.1152/jappl.1964.19.1.97. [DOI] [PubMed] [Google Scholar]
- 49.Sandhaus R. Pulmonary function in osteogenesis imperfecta. In: Osteogenesis Imperfecta: a Translational Approach to Brittle Bone Disease, edited by Shapiro J. Amsterdam: Elsevier, 2013, p. 335–341. [Google Scholar]
- 50.Shapiro JR, Burn VE, Chipman SD, Jacobs JB, Schloo B, Reid L, Larsen N, Louis F. Pulmonary hypoplasia and osteogenesis imperfecta type II with defective synthesis of alpha I(1) procollagen. Bone 10: 165–171, 1989. doi: 10.1016/8756-3282(89)90049-5. [DOI] [PubMed] [Google Scholar]
- 51.Sillence DO, Rimoin DL. Classification of osteogenesis imperfect. Lancet 311: 1041–1042, 1978. doi: 10.1016/S0140-6736(78)90763-8. [DOI] [PubMed] [Google Scholar]
- 52.Sillence DO, Rimoin DL, Danks DM. Clinical variability in osteogenesis imperfecta-variable expressivity or genetic heterogeneity. Birth Defects Orig Artic Ser 15, Suppl 5B: 113–129, 1979. [PubMed] [Google Scholar]
- 53.Sly PD, Collins RA, Thamrin C, Turner DJ, Hantos Z. Volume dependence of airway and tissue impedances in mice. J Appl Physiol (1985) 94: 1460–1466, 2003. doi: 10.1152/japplphysiol.00596.2002. [DOI] [PubMed] [Google Scholar]
- 54.Stanley NN, Alper R, Cunningham EL, Cherniack NS, Kefalides NA. Effects of a molecular change in collagen on lung structure and mechanical function. J Clin Invest 55: 1195–1201, 1975. doi: 10.1172/JCI108037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Südy R, Fodor GH, Dos Santos Rocha A, Schranc Á, Tolnai J, Habre W, Peták F. Different contributions from lungs and chest wall to respiratory mechanics in mice, rats, and rabbits. J Appl Physiol (1985) 127: 198–204, 2019. doi: 10.1152/japplphysiol.00048.2019. [DOI] [PubMed] [Google Scholar]
- 56.Suki B, Stamenović D, Hubmayr R. Lung parenchymal mechanics. Compr Physiol 1: 1317–1351, 2011. doi: 10.1002/cphy.c100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thibeault DW, Pettett G, Mabry SM, Rezaiekhaligh MM. Osteogenesis imperfecta Type IIA and pulmonary hypoplasia with normal alveolar development. Pediatr Pulmonol 20: 301–306, 1995. doi: 10.1002/ppul.1950200508. [DOI] [PubMed] [Google Scholar]
- 58.Thiele F, Cohrs CM, Flor A, Lisse TS, Przemeck GK, Horsch M, Schrewe A, Gailus-Durner V, Ivandic B, Katus HA, Wurst W, Reisenberg C, Chaney H, Fuchs H, Hans W, Beckers J, Marini JC, Hrabé de Angelis M. Cardiopulmonary dysfunction in the Osteogenesis imperfecta mouse model Aga2 and human patients are caused by bone-independent mechanisms. Hum Mol Genet 21: 3535–3545, 2012. doi: 10.1093/hmg/dds183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tolnai J, Szabari MV, Albu G, Maár BA, Parameswaran H, Bartolák-Suki E, Suki B, Hantos Z. Functional and morphological assessment of early impairment of airway function in a rat model of emphysema. J Appl Physiol (1985) 112: 1932–1939, 2012. doi: 10.1152/japplphysiol.00587.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Valli M, Barnes AM, Gallanti A, Cabral WA, Viglio S, Weis MA, Makareeva E, Eyre D, Leikin S, Antoniazzi F, Marini JC, Mottes M. Deficiency of CRTAP in non-lethal recessive osteogenesis imperfecta reduces collagen deposition into matrix. Clin Genet 82: 453–459, 2012. doi: 10.1111/j.1399-0004.2011.01794.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanoirbeek JA, Rinaldi M, De Vooght V, Haenen S, Bobic S, Gayan-Ramirez G, Hoet PH, Verbeken E, Decramer M, Nemery B, Janssens W. Noninvasive and invasive pulmonary function in mouse models of obstructive and restrictive respiratory diseases. Am J Respir Cell Mol Biol 42: 96–104, 2010. doi: 10.1165/rcmb.2008-0487OC. [DOI] [PubMed] [Google Scholar]
- 62.Warburton D, El-Hashash A, Carraro G, Tiozzo C, Sala F, Rogers O, De Langhe S, Kemp PJ, Riccardi D, Torday J, Bellusci S, Shi W, Lubkin SR, Jesudason E. Lung organogenesis. Curr Top Dev Biol 90: 73–158, 2010. doi: 10.1016/S0070-2153(10)90003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.White ES. Lung extracellular matrix and fibroblast function. Ann Am Thorac Soc 12, Suppl 1: S30–S33, 2015. doi: 10.1513/AnnalsATS.201406-240MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widmann RF, Bitan FD, Laplaza FJ, Burke SW, DiMaio MF, Schneider R. Spinal deformity, pulmonary compromise, and quality of life in osteogenesis imperfecta. Spine 24: 1673–1678, 1999. doi: 10.1097/00007632-199908150-00008. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Y, Horowitz JC, Naba A, Ambalavanan N, Atabai K, Balestrini J, Bitterman PB, Corley RA, Ding BS, Engler AJ, Hansen KC, Hagood JS, Kheradmand F, Lin QS, Neptune E, Niklason L, Ortiz LA, Parks WC, Tschumperlin DJ, White ES, Chapman HA, Thannickal VJ. Extracellular matrix in lung development, homeostasis and disease. Matrix Biol 73: 77–104, 2018. doi: 10.1016/j.matbio.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]