Abstract
Spinal cord injury (SCI) is an established risk factor for central sleep apnea. Acetazolamide (ACZ), a carbonic anhydrase inhibitor, has been shown to decrease the frequency of central apnea by inducing mild metabolic acidosis. We hypothesized that ACZ would decrease the propensity to develop hypocapnic central apnea and decrease the apneic threshold. We randomized 16 participants with sleep-disordered breathing (8 SCI and 8 able-bodied controls) to receive ACZ (500 mg twice a day for 3 days) or placebo with a 1-wk washout before crossing over to the other drug arm. Study nights included polysomnography and determination of the hypocapnic apneic threshold and CO2 reserve using noninvasive ventilation. For participants with spontaneous central apnea, CO2 was administered until central apnea was abolished, and CO2 reserve was measured as the difference in end-tidal Pco2 () before and after. Steady-state plant gain, the response of end-tidal Pco2 to changes in ventilation, was calculated from and V̇e ratio during stable sleep. Controller gain, the response of ventilatory drive to changes in end-tidal Pco2, was defined as the ratio of change in V̇e between control and hypopnea to the ΔCO2 during stable non-rapid eye movement sleep. Treatment with ACZ for three days resulted in widening of the CO2 reserve (−4.0 ± 1.2 vs. −3.0 ± 0.7 mmHg for able-bodied, −3.4 ± 1.9 vs. −2.2 ± 2.2 mmHg for SCI, P < 0.0001), and a corresponding decrease in the hypocapnic apnea threshold (28.3 ± 5.2 vs. 37.1 ± 5.6 mmHg for able-bodied, 29.9 ± 5.4 vs. 34.8 ± 6.9 mmHg for SCI, P < 0.0001), respectively. ACZ significantly reduced plant gain when compared with placebo (4.1 ± 1.7 vs. 5.4 ± 1.8 mmHg/L min for able-bodied, 4.1 ± 2.0 vs. 5.1 ± 1.7 mmHg·L−1·min for SCI, P < 0.01). Acetazolamide decreased apnea-hypopnea index (28.8 ± 22.9 vs. 39.3 ± 24.1 events/h; P = 0.05), central apnea index (0.6 ± 1.5 vs. 6.3 ± 13.1 events/h; P = 0.05), and oxyhemoglobin desaturation index (7.5 ± 8.3 vs. 19.2 ± 15.2 events/h; P = 0.01) compared with placebo. Our results suggest that treatment with ACZ decreases susceptibility to hypocapnic central apnea due to decreased plant gain. Acetazolamide may attenuate central sleep apnea and improve nocturnal oxygen saturation, but its clinical utility requires further investigation in a larger sample of patients.
NEW & NOTEWORTHY Tetraplegia is a risk factor for central sleep-disordered breathing (SDB) and is associated with narrow CO2 reserve (a marker of susceptibility to central apnea). Treatment with high-dose acetazolamide for 3 days decreased susceptibility to hypocapnic central apnea and reduced the frequency of central respiratory events during sleep. Acetazolamide may play a therapeutic role in alleviating central SDB in patients with cervical spinal cord injury, but larger clinical trials are needed.
Keywords: acetazolamide, central sleep apnea, loop gain, spinal cord injury
INTRODUCTION
Spinal cord injury (SCI) is a major cause of disability in the United States (1). Chronic SCI is associated with a number of complications detrimental to the quality of life. Respiratory complications represent major causes of morbidity and mortality in this population. Individuals with SCI experience a higher burden of sleep-disordered breathing (SDB) than the general population (36). In fact, tetraplegia may be a distinct risk factor for central sleep apnea (CSA) (24). Overall, SDB is a significant cause of morbidity and mortality in these patients (9).
Acetazolamide (ACZ), a carbonic anhydrase inhibitor, is a respiratory stimulant that has been used for the treatment of altitude sickness and CSA (2, 4, 18, 30). Acetazolamide acts by inhibiting luminal carbonic anhydrase, leading to excretion of bicarbonate, culminating in metabolic acidosis and stabilization of breathing (29, 30).
While ACZ has been investigated as a therapy for CSA in ambulatory patients, it has not been studied in individuals with SCI. On this basis, we hypothesized that acetazolamide would reduce the tendency of SCI patients to develop central apnea during sleep by reducing plant gain.
METHODS
Participants
The Institutional Review Board of the Wayne State University School of Medicine and the VA Medical Center approved the experimental protocol. Written informed consent was obtained, and subjects were screened by polysomnography. We enrolled 16 participants (8 able-bodied and 8 SCI) aged 59.5 ± 11.8 yr for the able-bodied group and 50.3 ± 12.8 yr for the SCI group with a body mass index (BMI) of 29.2 ± 1.9 kg/m2 for the able-bodied group and 25.8 ± 2.8 kg/m2 for the SCI group and evidence of sleep-disordered breathing [apnea-hypopnea index (AHI) ≥ 5 events/hour] (Table 1). Subjects either had chronic SCI (>6 mo) at or above the T6 level or were able-bodied control subjects. Subjects were excluded if they were less than 17 yr old or if they were pregnant or lactating. Subjects with sulfa drug allergies were excluded to avoid adverse reactions to acetazolamide.
Table 1.
Subject demographics and baseline sleep characteristics for all participants
| Able-Bodied | SCI | |
|---|---|---|
| N | 8 | 8 |
| Age, yr | 59.5 ± 11.8 | 50.3 ± 12.8 |
| Sex (M/F) | 6/2 | 7/1 |
| BMI, kg/m2 | 29.2 ± 1.9* | 25.8 ± 2.8* |
| NC, cm | 36.2 ± 9.6 | 38.2 ± 2.8 |
| Level of injury | ||
| Cervical | 7 | |
| Thoracic | 1 | |
| Time since injury, yr | 8.3 ± 4.7 | |
| ASIA Score | ||
| A | 1 | |
| B | 2 | |
| C | 1 | |
| D | 4 | |
| ESS | 11.0 ± 4.4 | 8.0 ± 3.2 |
| FSS | 3.6 ± 1.6 | 3.7 ± 1.8 |
| PSQI | 10.4 ± 3.5 | 10.8 ± 4.4 |
| AHI (events/h) | 21.3 ± 13.8 | 27.2 ± 32.0 |
| CAI (events/h) | 2.8 ± 4.5 | 7.3 ± 14.6 |
| OAI (events/h) | 1.7 ± 2.0 | 2.8 ± 3.7 |
| HI (events/h) | 17.0 ± 12.3 | 11.6 ± 7.9 |
| ODI (events/h) | 8.9 ± 13.0 | 19.9 ± 34.1 |
AHI, apnea-hypopnea index; CAI, central apnea index; ESS, Epworth Sleepiness Scale; FSS, Fatigue Severity Scale; HI, hypopnea index; NC, neck circumference; OAI, obstructive apnea index; ODI, oxyhemoglobin desaturation index; PSQI, Pittsburgh Sleep Quality Index. One able-bodied subject did not complete the baseline PSQI.
P < 0.05.
Study Procedures
If subjects had no prior clinical polysomnography (PSG), they were screened via either in-laboratory PSG or home-sleep testing. If subjects had undergone polysomnography before enrollment and were found to meet the inclusion criteria, they proceeded directly to randomization. Subjects were randomized to 3 days of oral acetazolamide (ACZ) 500 mg twice a day or placebo twice a day. After completing the first drug arm, subjects underwent a 1-wk washout period before crossing over to the other drug arm. On the third night of treatment for each drug arm, participants were assessed by in-laboratory PSG with electroencephalography, surface electromyography, and electrocardiography (Carefusion, SomnoStar z4 Sleep System, San Diego, CA) to determine AHI, central apnea index (CAI), and oxyhemoglobin desaturation index (ODI). To assess airflow, subjects were fitted with a nasal mask connected to an in-line pneumotachometer (Hans Rudolph; model 3700A, Shawnee, KS), from which data were acquired by an RSS 100HR Research Pneumotach System (Hans Rudolph). The end-tidal partial pressure of carbon dioxide ( was measured by connecting a tube placed in the nasal vestibule to a respiratory gas analyzer (CWE, Inc., GEMINI Respiratory Monitor, Ardmore, PA). Airflow and were recorded by a PowerLab data acquisition system (AD Instruments, Inc., model 16SP, Colorado Springs, CO). Tidal volume (VT) was derived by integrating the airflow channel, and minute ventilation (V̇e) was calculated by multiplying VT by breathing frequency (FB). Arterial oxygen saturation was monitored by an ear clip oximeter (Ohmeda Medical Inc., Biox 3740, Laurel, MD).
Hypocapnic apnea threshold and CO2 reserve protocol.
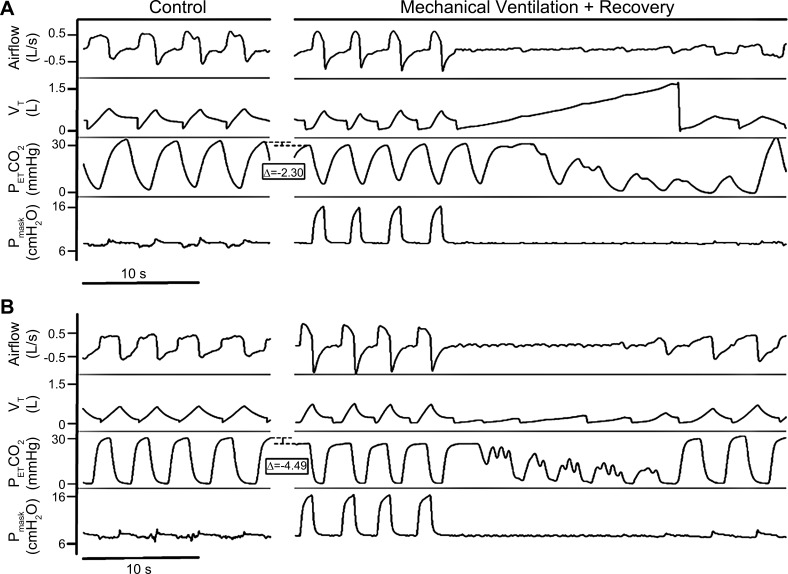
The hypocapnic apneic threshold (AT) was determined by the administration of noninvasive ventilation (NIV) during stable non-rapid eye movement (NREM) sleep in the supine position, as described previously (24). NIV (Philips Respironics, OmniLab Advanced+, Murrysville, PA) in bilevel PAP mode was used to induce hyperventilation by increasing the inspiratory positive airway pressure (IPAP) to a minimum of 8 cm H2O above expiratory positive airway pressure (EPAP). Hyperpnea was induced for 3 min until a sustained decrease in was observed, at which point the IPAP was reduced to equal EPAP for at least 3 min before commencing the next trial. IPAP was incrementally increased with each successive trial by 2 cmH2O until an induced hypocapnic central apnea resulted at the termination of the trial (Fig. 1).
Fig. 1.
Representative polygraphs from the noninvasive ventilation protocol depicting the difference in apneic threshold (AT) in a single spinal cord injury subject between the placebo-treated (A) and acetazolamide-treated (B) arms. Eupneic is represented by the upper dashed line of the bracket, and the AT is represented by the lower dashed line of the bracket. VT, tidal volume; , end-tidal CO2 partial pressure; Pmask, mask pressure.
If the subject experienced persistent spontaneous central apneas before beginning the intervention, a gas mixture containing 40% CO2 balanced with nitrogen was administered in 0.5 L/min increments at 5-min intervals to stabilize breathing (supplementary CO2 protocol). Once the amount of CO2 necessary to resolve central apneas was determined, was allowed to return to baseline, after which three 5-min trials at the therapeutic CO2, separated by sufficient time to return to baseline , were performed to confirm the effectiveness of the CO2. In these studies, the apneic threshold was defined as the at which apneas were eliminated.
Peripheral hyperoxic chemoresponsiveness protocol.
The contribution of the peripheral chemoreflex to maintaining eupnea was assessed by brief episodes of hyperoxia during stable non-REM sleep in the supine position, as described previously (25). Subjects underwent three successive 30-s hyperoxic exposures ( >40%) separated by 3-min recovery periods breathing room air.
Data Analysis
Baseline parameters.
Standard PSG was performed according to the American Academy of Sleep Medicine (AASM) standards, using the Carefusion Sleep System (SomnoStar z4, San Diego, CA). Respiratory events were scored per the 2012 AASM recommended scoring criteria (5). PSGs were scored by research staff who were certified by the AASM interscorer reliability program and blinded to the intervention, after which all PSGs were reviewed by a single sleep physician to ensure consistency. Baseline ventilatory parameters (VT, V̇e, FB, TI, TE, O2Sat, and ) were measured before the intervention as the average of 10 breaths during stable non-REM sleep. For subjects undergoing the NIV protocol, baseline breaths were selected from the period when the subject was connected to the breathing circuit with the ventilator set to CPAP mode at the minimum pressure setting for each subject (~4 cmH2O). For the subject that underwent the supplementary CO2 protocol, baseline breaths were selected from the period before the administration of CO2. Steady-state plant gain was calculated in each participant from the baseline -V̇e ratio during stable non-REM sleep, as described recently (24).
Apneic threshold measurement.
noninvasive ventilation protocol.
Data were analyzed to determine the apneic threshold as described in Ref. 27. For the NIV protocol, five breaths selected before increasing IPAP were designated as the control breaths, and the last five breaths of the NIV period were designated the mechanical ventilation breaths. The hypocapnic apnea threshold for the NIV protocol was defined as the highest average during the mechanical ventilation breaths that resulted in an apnea following the return to baseline pressure. The CO2 reserve (∆CO2) was defined as the difference between average for the five control breaths and for the last five breaths before the cessation of NIV.
hypercapnia protocol.
The apnea threshold was defined as the minimum , at which spontaneous central apneas were eliminated, as described previously (24). For this protocol, the ∆CO2 was defined as Δ between the control period (stable non-REM sleep after central apnea is eliminated) and the last five breaths before central apnea.
calculation of controller gain.
For both protocols, controller gain was calculated as the difference between control V̇e and postmechanical ventilation V̇e divided by the difference between control and the at the end of mechanical ventilation for the trial that produced the hypocapnic apnea threshold.
Determination of peripheral hyperoxic chemoresponsiveness.
For each determination of the peripheral chemoresponsiveness, the hyperoxic ventilatory response (HOV) was calculated as the difference in V̇e between the nadir breath during the hyperoxia period and eupneic ventilation.
Statistical Methods
Demographic characteristics were summarized as means and SD or frequency and percentage scores, as applicable. Demographics of the able-bodied and SCI groups were compared using two-tailed independent samples t tests. One-tailed paired samples t tests were used to determine the change in sleep parameters, including AHI, CAI, ODI, respiratory effort-related arousal index (RERAI), periodic leg movement arousal index (PLMAI), and sleep efficiency compared with placebo. Ventilatory and physiological parameters, including VT, V̇e, FB, TI, TE, TTot, O2Sat, and for the placebo and acetazolamide arms were compared using two-tailed paired sample t tests. A repeated-measures ANOVA was used to examine the change in CO2 reserve, controller gain, plant gain, and HOV within participants, between groups (able-bodied and SCI), and within participants by between-group interactions. Correlation analysis was conducted to determine the relationship between change in CO2 reserve and the three main SDB parameters (AHI, CAI, and ODI). The significance level was set at a P value equal to or less than 0.05. All statistical analyses were carried out using SPSS version 25 (IBM, Armonk, NY). Power analysis for the sample size of n = 16 resulted in 80% power to detect a change of 0.60 SD at a 0.05 level of significance. The PASS computer software (version 11, Hintze, 2011) was used to estimate the required sample size.
RESULTS
We studied 16 participants with SDB (8 SCI and 8 able-bodied) in the supine position during NREM sleep. All 16 subjects completed both the hyperoxia and apneic threshold protocols on both acetazolamide and placebo. The NIV protocol for determining the CO2 reserve was employed for all eight able-bodied subjects and seven SCI subjects, with the remaining SCI subject undergoing the supplemental CO2 protocol. This participant was not included in the analysis for controller gain and steady-state plant gain, as calculation of these parameters has not been validated in the context of supplemental CO2. Demographics and sleep characteristics from baseline polysomnography of the able-bodied and SCI groups are shown in Table 1. Consumption of study medication was confirmed by measuring total CO2 levels, which decreased on ACZ versus placebo (22.6 ± 2.4 vs. 29.0 ± 3.1 mEq/L, P < 0.0001). Ventilatory parameters for the combined able-bodied and SCI groups on placebo and ACZ are summarized in Table 2.
Table 2.
Ventilatory parameters of participants after three days of placebo versus acetazolamide treatment
| Able-Bodied | SCI | |||
|---|---|---|---|---|
| Placebo | Acetazolamide | Placebo | Acetazolamide | |
| Total CO2, mEq/L | 29.3 ± 3.7 | 22.4 ± 2.6* | 28.7 ± 2.6 | 22.7 ± 2.3* |
| V̇e, L/min | 7957.2 ± 2271.6 | 9123.9 ± 3204.4 | 6589.0 ± 2373.9 | 7939.6 ± 3134.8 |
| VT, L | 522.1 ± 104.1 | 584.5 ± 158.2 | 501.1 ± 30.7 | 569.4 ± 164.4 |
| FB, breaths/min | 15.3 ± 3.2 | 15.5 ± 3.1 | 13.0 ± 2.8 | 13.8 ± 3.8 |
| TI, s | 1.8 ± 0.3 | 1.8 ± 0.3 | 2.0 ± 0.3 | 2.0 ± 0.5 |
| TE, s | 2.3 ± 0.6 | 2.3 ± 0.6 | 2.9 ± 0.6 | 2.7 ± 1.0 |
| TTot, s | 4.1 ± 0.9 | 4.0 ± 0.8 | 4.8 ± 0.9 | 4.7 ± 1.4 |
| TI/TTot | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.4 ± 0.0 | 0.4 ± 0.1 |
| , mmHg | 37.4 ± 3.0 | 32.1 ± 5.3 | 38.1 ± 7.0 | 33.9 ± 6.0 |
| O2Sat, % | 96.3 ± 1.0 | 97.7 ± 1.1 | 96.1 ± 3.2 | 97.2 ± 1.9 |
FB, respiratory rate; O2Sat, oxyhemoglobin saturation; PETCO2, end tidal CO2 partial pressure; TE, expiratory duration; TI, inspiratory duration; TI/TTot, fractional inspiratory time; TTot, breath duration; V̇e, minute ventilation; VT, tidal volume.
P < 0.05 vs. placebo using paired-samples t test.
Physiological Outcomes
Effect of acetazolamide on hypocapnic apnea threshold.
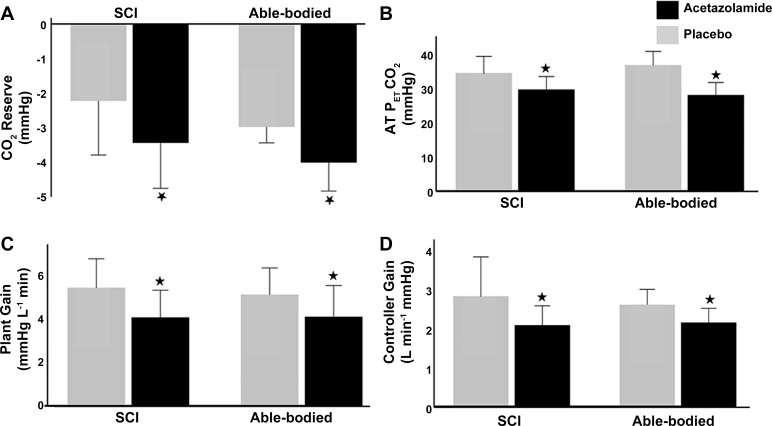
CO2 reserve was widened significantly for both able-bodied and SCI groups on acetazolamide compared with placebo, as shown in Fig. 2A (−4.0 ± 1.2 vs. −3.0 ± 0.7 mmHg for able-bodied, −3.4 ± 1.9 vs −2.2 ± 2.2 mmHg for SCI, F = 36.95, P < 0.0001). There was no significant difference in the effect size of acetazolamide in able-bodied and SCI individuals (F = 0.25, P = 0.62). The widening of the CO2 reserve on acetazolamide was accompanied by a significant decrease in AT-CO2 from 37.1 ± 5.6 mmHg to 28.3 ± 5.2 mmHg in the able-bodied group and from 34.8 ± 6.9 mmHg to 29.9 ± 5.4 mmHg in the SCI group (F = 83.34, P < 0.0001) (Fig. 2B). There was no significant difference in effect size between the two groups (F = 0.10, P = 0.76). Acetazolamide consumption significantly reduced steady-state plant gain compared with placebo for the SCI group as shown in Fig. 2C (4.1 ± 1.7 vs. 5.4 ± 1.8 mmHg·L−1·min−1) and the able-bodied group (4.1 ± 2.0 vs. 5.1 ± 1.7 mmHg·L−1·min, F = 19.61, P < 0.01), but there was no significant difference in the effect size between the able-bodied and SCI groups (F = 0.41, P = 0.53). Controller gain was significantly reduced on ACZ compared with placebo in the SCI group (2.1 ± 0.7 vs. 2.8 ± 1.3 L·min−1·mmHg−1) and the able-bodied group (2.2 ± 0.5 vs. 2.6 ± 0.6, F = 8.48, P = 0.01). However, the difference in the effect size of acetazolamide treatment between the able-bodied and SCI groups was not statistically significant (F = 0.49, P = 0.50) (Fig. 2D).
Fig. 2.
Effect of acetazolamide on CO2 reserve (n = 16) (A), hypocapnic apnea threshold (n = 16) (B), plant gain (n = 16) (C), and controller gain (D) (n = 15; one subject excluded as described in methods). *P < 0.05 vs. placebo using repeated-measures ANOVA. AT , end-tidal partial pressure of CO2 at the hypocapnic apnea threshold.
Effect of acetazolamide on peripheral hyperoxic chemoresponsiveness.
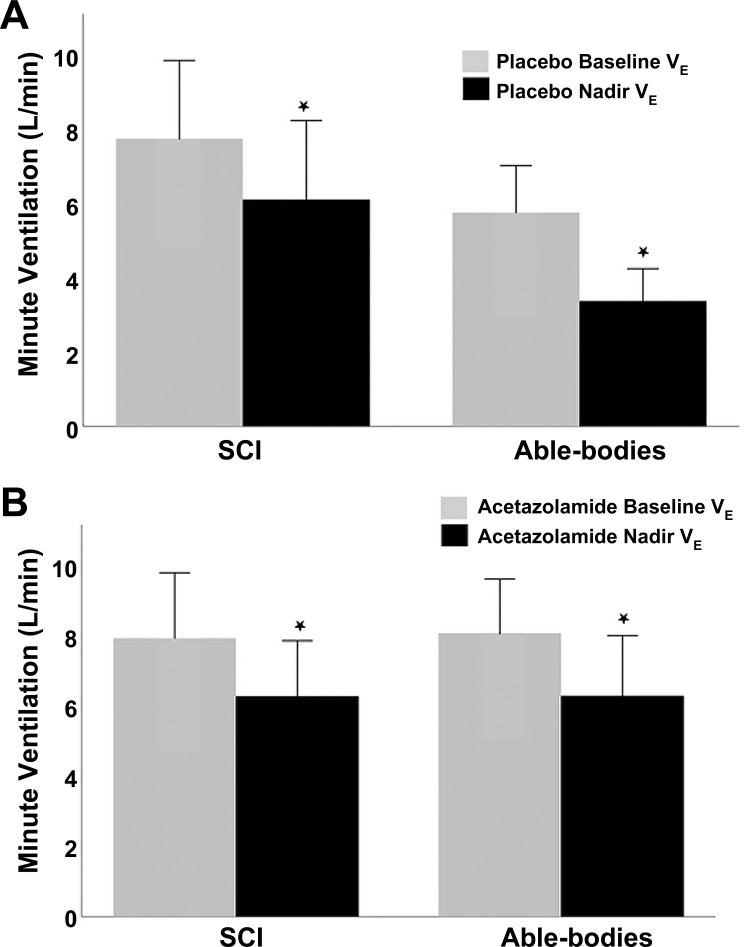
Hyperoxic exposure resulted in a significant decrease in V̇e in both groups (F = 86.75, P < 0.0001) for both the placebo (7.8 ± 3.0 to 6.1 ± 3.0 L/min for able-bodied, 5.8 ± 1.8 to 3.4 ± 1.2 L/min for SCI, Fig. 3A) and acetazolamide arms (7.9 ± 2.6 to 6.3 ± 2.2 L/min for able-bodied, 8.0 ± 2.2 to 6.3 ± 2.4 L/min for SCI, Fig. 3B). There was no significant interaction between the groups and drug arms (P = 0.45).
Fig. 3.
Hyperoxic ventilatory response (HOV) in the able-bodied and SCI groups on placebo (A) and acetazolamide (B) (n = 16). *P < 0.05 for nadir versus baseline using repeated measures ANOVA. V̇e, minute ventilation.
Clinical Outcomes
Effect of acetazolamide on sleep-disordered breathing and sleep parameters.
To explore a potential therapeutic effect of acetazolamide in treating CSA, we obtained pilot data relating to the impact of acetazolamide on CSA indices (Table 3) Significant decreases were observed on acetazolamide compared with placebo in AHI (28.8 ± 22.9 vs. 39.3 ± 24.1 events/h, respectively, F = 3.00, P = 0.05), CAI (0.6 ± 1.5 vs. 6.3 ± 13.1 events/h, respectively, F = 3.16, P = 0.05) and ODI (7.5 ± 8.3 vs. 19.2 ± 15.2 events/h, respectively, F = 6.28, P = 0.01). There was no correlation between changes in any of these three parameters and changes in CO2 reserve. PLMAI was slightly increased on acetazolamide compared with placebo (1.1 ± 1.7 vs. 0.3 ± 0.5 events/h, respectively, F = 3.07, P = 0.05) Acetazolamide use was not associated with significant differences in sleep efficiency or respiratory effort-related arousal index.
Table 3.
Clinical sleep parameters for all subjects on placebo and acetazolamide
| Placebo | Acetazolamide | |
|---|---|---|
| Sleep efficiency, % | 83.5 ± 10.6 | 79.1 ± 12.2 |
| PLMAI (events/h) | 0.3 ± 0.5 | 1.1 ± 1.7* |
| RERAI (events/h) | 23.9 ± 21.8 | 21.0 ± 17.3 |
| AHI (events/h) | 39.3 ± 24.1 | 28.8 ± 22.9* |
| CAI (events/h) | 6.3 ± 13.1 | 0.6 ± 1.5* |
| ODI (events/h) | 19.2 ± 15.2 | 7.5 ± 8.3* |
AHI, apnea-hypopnea index; CAI, central apnea index; ODI, oxyhemoglobin desaturation index PLMAI, periodic leg movement arousal index; RERAI, respiratory effort-related arousal index.
P < 0.05 vs. placebo using one-tailed, paired-samples t test.
DISCUSSION
The results of our study corroborate and extend existing literature on the utility of ACZ in the context of SDB. The two key findings of our study were 1) oral acetazolamide therapy for 3 days is sufficient to significantly reduce plant gain and widen the CO2 reserve in both able-bodied patients and those with chronic SCI, and 2) these changes are accompanied by reductions in several measures of SDB severity, including AHI, CAI, and ODI.
Methodological Considerations
Several aspects of the study should be considered when interpreting the results. First, several steps were taken to enhance adherence to therapy. Participants were contacted each of the 3 days on medication to ensure compliance, and blood samples were drawn on the third night of each drug arm to confirm that total CO2 decreased compared with placebo. Second, the 3-day dosing regimen was sufficient to produce the desired metabolic acidosis. The duration was based on a study by Teppema and Dehan, who demonstrated that 3 days of acetazolamide was sufficient to produce metabolic acidosis and increase minute ventilation (30). The dosage was selected on the basis of previous studies that used 500 mg twice a day (3, 13). The Teppema and Dehan study used an ACZ dose of 250 mg three times a day, but we elected to employ the two-dose schedule to improve compliance. A potential complication is that a dosage of 1,000 mg/day may be large enough to inhibit erythrocyte carbonic anhydrase, reducing CO2 delivery to the lungs. This reduced CO2 delivery to the lungs may create an alveolar-arterial (A-a) gradient, which would cause the end-tidal CO2 measurement to underestimate the arterial CO2. Future studies should include arterial CO2 measurements to determine whether or not this dose alters the A-a gradient. Third, the washout period was set at 1 wk to ensure no contamination between drug arms. One week has been the washout duration employed by several previous studies using the same or comparable dosing regimen as our study (12, 13, 31).
Confounding Factors
Our study was not without confounding variables, the most noteworthy being discrepancies in the BMI between the SCI group and the able-bodied controls and the disparity between the number of male and female participants in both groups. The lower BMI in the SCI group is likely due to atrophy and wasting from the injury and would be difficult to avoid in any comparison between healthy individuals and those with SCI. It is not apparent what the significance of this discrepancy may be. Additionally, the sample we studied comprised primarily male subjects (13 men, 3 women). This may limit the applicability of these findings to female subjects, and further studies should include more female participants. However, epidemiological studies have demonstrated the paucity of central apnea in women (7). This scarcity was corroborated by physiological studies demonstrating a wider CO2 reserve in premenopausal women relative to men (35).
Effect of Acetazolamide on Susceptibility to Induced Central Apnea
The major finding of our study was that treatment with acetazolamide widened the CO2 reserve and, hence, decreased the propensity to develop central apnea, with a corresponding decrease in the frequency of central respiratory events. This effect was noted in able-bodied and individuals with SCI.
Our findings also provide significant insight into the mechanisms underlying the widening of the CO2 reserve. Specifically, acetazolamide was associated with decreased plant gain and controller gain but had no effect on peripheral chemoreceptor activity, thereby implicating a central mechanism of effect.
Our results and existing literature suggest several potential mechanistic explanations for the widening of the CO2 reserve observed following administration of ACZ. We found a 1.2 L/min increase in baseline V̇e on acetazolamide compared with placebo, confirming the respiratory stimulation effect of acetazolamide. Widening of the CO2 reserve was accompanied by decreased plant gain in both groups. It is of note that plant gain is elevated in patients with SCI, owing to reduced lung volumes (10, 28). Thus, ACZ-induced respiratory stimulation and reduction in plant gain occurs independently of lung volumes and pretreatment plant gain (10, 28). Accordingly, decreasing plant gain may dampen breath-to-breath oscillations in and stabilize respiration.
Decreased plant gain following acetazolamide administration may decrease overall loop gain, as previously demonstrated by Edwards et al. (13). However, our study differs from previous studies in the non-SCI population by demonstrating decreased controller gain (3, 13, 23, 30, 33). Conversely, Javaheri et al. (19) demonstrated increased controller gain following ACZ administration in patients with congestive heart failure. The reason for the discrepancy in findings is unclear but may reflect differences in the study population, acetazolamide dose, or methodological differences. One possible explanation is enhanced cerebrovascular reactivity with acetazolamide, leading to blunting of the ventilatory response slope to change in CO2 (see below). Other potential mechanisms include an interaction between the chemoreceptors and the sympathetic nervous system (6, 22). Regardless of the mechanism, decreased controller gain following acetazolamide administration may further stabilize respiration by mitigating the propensity to develop central apnea.
Acetazolamide administration has been shown to increase cerebrovascular responsiveness (CVR), which stabilizes breathing by improving the response time of the respiratory circuit to changes in (16, 17, 20, 32, 34). ACZ increased CVR to CO2 at both sea level and also at high altitude during wakefulness and enhanced brain-blood flow responses to CO2, in addition to increasing resting minute ventilation (14). After ACZ administration, cerebral blood flow (CBF) continues to respond to ventilation‐induced changes and to changes in blood pressure, which may have a complex end result on CBF and ventilatory control (15). Previous studies have demonstrated a link between cerebral blood flow and its response to CO2 (cerebrovascular CO2 reactivity), and ventilatory stability (14, 15, 34). Fan et al. (14) demonstrated that ACZ increased CBF and cerebrovascular hypocapnic and hypercapnic reactivity with associated blunting of the ventilatory slope to CO2 below eupnea, and with increased breathing stability, as reflected by reduced variability of V̇e, VT, and incidence of breathing oscillations. Moreover, the improvement in breathing patterns following ACZ administration occurred independently of any measurable changes in the sensitivity of the central or peripheral chemoreflex leading them to conclude that -mediated elevations in CBF and CVR may contribute to the stabilization of breathing following ACZ consumption. Thus, on the basis of the above-published findings, we speculate that one of the mechanisms of ventilatory stability with ACZ in our study may be mediated via enhanced CVR reactivity. Future studies should include measurements of cerebral blood flow to determine whether cerebral blood flow plays a role in the efficacy of acetazolamide treatment in CSA.
In summary, we have demonstrated that acetazolamide reduced susceptibility to hypocapnic central apnea without altering peripheral chemoresponsiveness, suggesting that the acetazolamide attenuates propensity to develop central apnea, in part, by a central chemoreceptor-dependent mechanism. Decreased plant gain may stabilize baseline respiration and decreased controller gain may dampen posthyperventilation ventilatory decline.
Clinical Implications
Widening of the CO2 reserve in our study was associated with reductions in the frequency of central respiratory events. Although the study was not designed to test a clinically meaningful therapeutic effect, our findings provide sufficient rationale to perform a clinical trial, testing the effectiveness of acetazolamide as a potential therapy for central SDB. The dual effect of ACZ on plant gain and controller gain suggests that ACZ may be beneficial in the central apnea of different mechanisms.
Our study has shown the efficacy of acetazolamide in reducing the severity of CSA and nocturnal oxyhemoglobin desaturation in both able-bodied and SCI participants. Acetazolamide has shown utility as a respiratory stimulant in the context of altitude sickness and central sleep apnea syndrome (2, 4, 18, 30). The current standard of therapy for central sleep apnea centers on positive pressure therapy in different forms, such as continuous or bilevel PAP or adaptive servo-ventilation, as well as supplemental oxygen (2, 8, 11, 21). Pharmacological therapy may be particularly appealing to patients with spinal cord injuries who have difficulty with PAP acceptance and tolerance (26). Therefore, acetazolamide may be a valuable treatment for the management of CSA in patients who do not tolerate device-based therapies.
In summary, we have found that acetazolamide decreases the propensity to central apnea via decreased loop gain, with the corresponding decrease in the indices of central SDB. Our findings support the need for a definitive clinical trial testing the effectiveness of acetazolamide as a pharmacologic therapy for central apnea.
GRANTS
Dr. M. S. Badr was supported by the Department of Veterans Affairs, Merit Review #1I01CX001040 and National Heart, Lung, and Blood Institute of Health #R01HL-130552. Dr. Sankari was supported by a Career Development Award from the (US) Department of Veterans Affairs Office of Research and Development #IK2CX-000547, #RX-002885 and from the National Heart, Lung, and Blood Institute Awards #HL-140447 and #HL-130552.
DISCLOSURES
This was not an industry-supported study. The authors have indicated no financial conflicts of interest.
AUTHOR CONTRIBUTIONS
Conception and design: A. Sankari and M.S.B.; analysis and interpretation: G.G., A. Sankari, M.E., H.O. H.Y., S.C., A. Salloum, and M.S.B.; drafting the manuscript: G.G., A. Sankari, and M.S.B.; preparing figures: G.G., A. Sankari, M.E., and M.S.B.
REFERENCES
- 1.National Spinal Cord Injury Statistical Center Spinal Cord Injury: Facts and Figures at a Glance (2019 SCI Data Sheet) Birmingham, AL: University of Alabama at Birmingham, 2019; https://www.nscisc.uab.edu/Public/Facts%20and%20Figures%202019%20-%20Final.pdf. [Google Scholar]
- 2.Aurora RN, Chowdhuri S, Ramar K, Bista SR, Casey KR, Lamm CI, Kristo DA, Mallea JM, Rowley JA, Zak RS, Tracy SL. The treatment of central sleep apnea syndromes in adults: practice parameters with an evidence-based literature review and meta-analyses. Sleep (Basel) 35: 17–40, 2012. doi: 10.5665/sleep.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bashir Y, Kann M, Stradling JR. The effect of acetazolamide on hypercapnic and eucapnic/poikilocapnic hypoxic ventilatory responses in normal subjects. Pulm Pharmacol 3: 151–154, 1990. doi: 10.1016/0952-0600(90)90046-L. [DOI] [PubMed] [Google Scholar]
- 4.Basnyat B, Gertsch JH, Holck PS, Johnson EW, Luks AM, Donham BP, Fleischman RJ, Gowder DW, Hawksworth JS, Jensen BT, Kleiman RJ, Loveridge AH, Lundeen EB, Newman SL, Noboa JA, Miegs DP, O’Beirne KA, Philpot KB, Schultz MN, Valente MC, Wiebers MR, Swenson ER. Acetazolamide 125 mg BD is not significantly different from 375 mg BD in the prevention of acute mountain sickness: the prophylactic acetazolamide dosage comparison for efficacy (PACE) trial. High Alt Med Biol 7: 17–27, 2006. doi: 10.1089/ham.2006.7.17. [DOI] [PubMed] [Google Scholar]
- 5.Berry R, Brooks R, Gamaldo C, Harding S, Marcus C, Vaughn B. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications, version 2.0 Darien, Illinois: American Academy of Sleep Medicine, 2012. [Google Scholar]
- 6.Biering-Sørensen F, Biering-Sørensen T, Liu N, Malmqvist L, Wecht JM, Krassioukov A. Alterations in cardiac autonomic control in spinal cord injury. Auton Neurosci 209: 4–18, 2018. doi: 10.1016/j.autneu.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 7.Bixler EO, Vgontzas AN, Lin HM, Ten Have T, Rein J, Vela-Bueno A, Kales A. Prevalence of sleep-disordered breathing in women: effects of gender. Am J Respir Crit Care Med 163: 608–613, 2001. doi: 10.1164/ajrccm.163.3.9911064. [DOI] [PubMed] [Google Scholar]
- 8.Bordier P, Lataste A, Hofmann P, Robert F, Bourenane G. Nocturnal oxygen therapy in patients with chronic heart failure and sleep apnea: a systematic review. Sleep Med 17: 149–157, 2016. doi: 10.1016/j.sleep.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 9.Cardozo CP. Respiratory complications of spinal cord injury. J Spinal Cord Med 30: 307–308, 2007. doi: 10.1080/10790268.2007.11753945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deacon-Diaz NL, Sands SA, McEvoy RD, Catcheside PG. Daytime loop gain is elevated in obstructive sleep apnea but not reduced by CPAP treatment. J Appl Physiol 125: 1490–1497, 2018. doi: 10.1152/japplphysiol.00175.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dohi T, Kasai T, Narui K, Ishiwata S, Ohno M, Yamaguchi T, Momomura S. Bi-level positive airway pressure ventilation for treating heart failure with central sleep apnea that is unresponsive to continuous positive airway pressure. Circ J 72: 1100–1105, 2008. doi: 10.1253/circj.72.1100. [DOI] [PubMed] [Google Scholar]
- 12.Edwards BA, Connolly JG, Campana LM, Sands SA, Trinder JA, White DP, Wellman A, Malhotra A. Acetazolamide attenuates the ventilatory response to arousal in patients with obstructive sleep apnea. Sleep (Basel) 36: 281–285, 2013. doi: 10.5665/sleep.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards BA, Sands SA, Eckert DJ, White DP, Butler JP, Owens RL, Malhotra A, Wellman A. Acetazolamide improves loop gain but not the other physiological traits causing obstructive sleep apnoea. J Physiol 590: 1199–1211, 2012. doi: 10.1113/jphysiol.2011.223925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fan JL, Burgess KR, Thomas KN, Lucas SJ, Cotter JD, Kayser B, Peebles KC, Ainslie PN. Effects of acetazolamide on cerebrovascular function and breathing stability at 5050 m. J Physiol 590: 1213–1225, 2012. doi: 10.1113/jphysiol.2011.219923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fierstra J, Sobczyk O, Battisti-Charbonney A, Mandell DM, Poublanc J, Crawley AP, Mikulis DJ, Duffin J, Fisher JA. Measuring cerebrovascular reactivity: what stimulus to use? J Physiol 591: 5809–5821, 2013. doi: 10.1113/jphysiol.2013.259150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friberg L, Kastrup J, Rizzi D, Jensen JB, Lassen NA. Cerebral blood flow and end-tidal Pco2 during prolonged acetazolamide treatment in humans. Am J Physiol Heart Circ Physiol 258: H954–H959, 1990. doi: 10.1152/ajpheart.1990.258.4.H954. [DOI] [PubMed] [Google Scholar]
- 17.Hauge A, Nicolaysen G, Thoresen M. Acute effects of acetazolamide on cerebral blood flow in man. Acta Physiol Scand 117: 233–239, 1983. doi: 10.1111/j.1748-1716.1983.tb07202.x. [DOI] [PubMed] [Google Scholar]
- 18.Javaheri S. Acetazolamide improves central sleep apnea in heart failure: a double-blind, prospective study. Am J Respir Crit Care Med 173: 234–237, 2006. doi: 10.1164/rccm.200507-1035OC. [DOI] [PubMed] [Google Scholar]
- 19.Javaheri S, Sands SA, Edwards BA. Acetazolamide attenuates Hunter-Cheyne-Stokes breathing but augments the hypercapnic ventilatory response in patients with heart failure. Ann Am Thorac Soc 11: 80–86, 2014. doi: 10.1513/AnnalsATS.201306-201OC. [DOI] [PubMed] [Google Scholar]
- 20.Lassen NA, Friberg L, Kastrup J, Rizzi D, Jensen JJ. Effects of acetazolamide on cerebral blood flow and brain tissue oxygenation. Postgrad Med J 63: 185–187, 1987. doi: 10.1136/pgmj.63.737.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pepperell JC, Maskell NA, Jones DR, Langford-Wiley BA, Crosthwaite N, Stradling JR, Davies RJ. A randomized controlled trial of adaptive ventilation for Cheyne-Stokes breathing in heart failure. Am J Respir Crit Care Med 168: 1109–1114, 2003. doi: 10.1164/rccm.200212-1476OC. [DOI] [PubMed] [Google Scholar]
- 22.Ponikowski P, Banasiak W. Chemosensitivity in chronic heart failure. Heart Fail Monit 1: 126–131, 2001. [PubMed] [Google Scholar]
- 23.Pranathiageswaran S, Badr MS, Nickert N, Chowdhuri S. Effect of acetazolamide on chemosensitivity during NREM sleep. In: B60 New Insights in Pathophysiology, Epidemiology, and Detection of Sleep-Disordered Breathing. New York: American Thoracic Society, 2015, p. A3547–A3547. [Google Scholar]
- 24.Sankari A, Bascom AT, Chowdhuri S, Badr MS. Tetraplegia is a risk factor for central sleep apnea. J Appl Physiol (1985) 116: 345–353, 2014. doi: 10.1152/japplphysiol.00731.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sankari A, Bascom AT, Riehani A, Badr MS. Tetraplegia is associated with enhanced peripheral chemoreflex sensitivity and ventilatory long-term facilitation. J Appl Physiol (1985) 119: 1183–1193, 2015. doi: 10.1152/japplphysiol.00088.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sankari A, Vaughan S, Bascom A, Martin JL, Badr MS. Sleep-disordered breathing and spinal cord injury: a state-of-the-art review. Chest 155: 438–445, 2019. doi: 10.1016/j.chest.2018.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sankri-Tarbichi AG, Grullon K, Badr MS. Effects of clonidine on breathing during sleep and susceptibility to central apnoea. Respir Physiol Neurobiol 185: 356–361, 2013. doi: 10.1016/j.resp.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stepp EL, Brown R, Tun CG, Gagnon DR, Jain NB, Garshick E. Determinants of lung volumes in chronic spinal cord injury. Arch Phys Med Rehabil 89: 1499–1506, 2008. doi: 10.1016/j.apmr.2008.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swenson ER. Carbonic anhydrase inhibitors and ventilation: a complex interplay of stimulation and suppression. Eur Respir J 12: 1242–1247, 1998. doi: 10.1183/09031936.98.12061242. [DOI] [PubMed] [Google Scholar]
- 30.Teppema LJ, Dahan A. Acetazolamide and breathing. Does a clinical dose alter peripheral and central CO2 sensitivity? Am J Respir Crit Care Med 160: 1592–1597, 1999. doi: 10.1164/ajrccm.160.5.9903088. [DOI] [PubMed] [Google Scholar]
- 31.Ulrich S, Keusch S, Hildenbrand FF, Lo Cascio C, Huber LC, Tanner FC, Speich R, Bloch KE. Effect of nocturnal oxygen and acetazolamide on exercise performance in patients with pre-capillary pulmonary hypertension and sleep-disturbed breathing: randomized, double-blind, cross-over trial. Eur Heart J 36: 615–623, 2015. doi: 10.1093/eurheartj/eht540. [DOI] [PubMed] [Google Scholar]
- 32.Vorstrup S, Henriksen L, Paulson OB. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest 74: 1634–1639, 1984. doi: 10.1172/JCI111579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.White DP, Zwillich CW, Pickett CK, Douglas NJ, Findley LJ, Weil JV. Central sleep apnea. Improvement with acetazolamide therapy. Arch Intern Med 142: 1816–1819, 1982. doi: 10.1001/archinte.1982.00340230056012. [DOI] [PubMed] [Google Scholar]
- 34.Xie A, Skatrud JB, Barczi SR, Reichmuth K, Morgan BJ, Mont S, Dempsey JA. Influence of cerebral blood flow on breathing stability. J Appl Physiol (1985) 106: 850–856, 2009. doi: 10.1152/japplphysiol.90914.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou XS, Shahabuddin S, Zahn BR, Babcock MA, Badr MS. Effect of gender on the development of hypocapnic apnea/hypopnea during NREM sleep. J Appl Physiol (1985) 89: 192–199, 2000. doi: 10.1152/jappl.2000.89.1.192. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the respiratory system: basic research and current clinical treatment options. J Spinal Cord Med 30: 319–330, 2007. doi: 10.1080/10790268.2007.11753947. [DOI] [PMC free article] [PubMed] [Google Scholar]