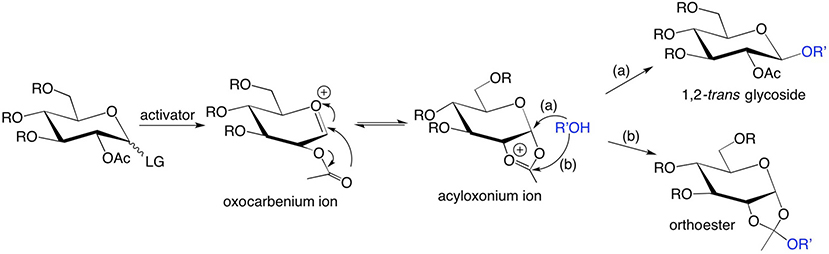
Scheme 1.
Formation of (a) a 1,2-trans-glycoside via intramolecular rearrangement of an oxocarbenium ion intermediate involving the acyl oxygens at C2, followed by SN2 ring-opening of an acyloxonium ion intermediate involving R’OH, and (b) an orthoester via nucleophilic attack of the acyloxonium ion intermediate by R’OH.