Abstract
Acute progesterone injection has been shown to reduce brain edema following traumatic brain injury (TBI) due to its neuroprotective effect. We investigated the effects of sustained release of progesterone through implantation of subcutaneous capsules on rat's brain edema and alteration of cerebrospinal fluid (CSF), and serum ratio of NGF/IL-6 after TBI. This experiment was performed on ovariectomized (OVX) rats and the brain injury was induced by Marmarou's method. A high and a low dose of progesterone (HP and LP) was injected intraperitoneally two h after the brain injury. In addition, in the capsule progesterone-treated group (CP), the intervention was implemented 6 h after the brain injury. Brain edema, NGF and IL-6 biomarkers in serum and cerebrospinal fluid (CSF) were measured 48 h after the TBI in injection groups and one week after the TBI in the CP group. No significant difference was found in the two groups or in the admonition methods. After TBI, the NGF level increased and IL-6 level decreased by injection of both doses, as well as by taking the capsule. Ratio of NGF/IL-6 in CSF increased significantly by all forms of progesterone administration. The increase in the level of NGF and IL-6 after TBI was higher in CSF than in serum. These results indicated that effects of progesterone in capsule form were better than the injection form. Progesterone probably works by increasing NGF and reducing IL-6. Future studies should investigate the ratio of these biomarkers as a variable to determine the neuroprotective effects of another drug.
Keywords: Neuroscience, Endocrinology, Health sciences, Physiology, Nervous system, Reproductive system, Progesterone, Traumatic brain injury, Brain edema, IL-6, NGF/IL-6
Neuroscience; Endocrinology; Health sciences; Physiology; Nervous system; Reproductive system; Progesterone; Traumatic brain injury; Brain edema; IL-6; NGF/IL-6
1. Introduction
Traumatic brain injury (TBI) is often referred to as the “silent epidemic” [1]. Current estimations indicate that, about 5.48 million people suffer from TBI each year [2] and its mortality increases with age [3].
It is been shown that, progesterone exerts its neuroprotective effects by reducing brain edema through following mechanisms: it reduces the permeability of blood-brain barrier (BBB) [4], aquaporin-4 (AQP4) expression [5], and intracranial pressure (ICP) [6]. It also increases cerebral perfusion pressure (CPP) [6] and reduces inflammation by decreasing serum intercellular, molecule-1 (ICAM-1) adhesion [7]. It has also been reported that following TBI, serum level of interleukin-6 (IL-6) and nerve growth factor (NGF) would increase [8]. IL-6 is a cytokine that is produced (in addition to macrophages) in neutrophils, fibroblasts, microglia, endothelial cells, and astrocytes in response to infection and other cytokines [9]. It is known that increased IL-6 level in TBI-induced inflammatory responses occurs in animal models and is one of the important factors in the development of responses in acute phase of TBI [10]. The progesterone decreases the expression of IL-6, which indicates a decrease in the process of acute inflammation [11].
NGF belongs to the neurotrophic family, which are involved in the regulation of growth, protection, proliferation, and survival of neurons [12]. During the inflammatory process, NGF is largely secreted from astrocytes and induces axonal growth [13].
Previous studies indicate that IL-8 [14], IL-1β, and tumor necrosis factor-α (TNF-α) are involved in the promotion of NGF synthesis following brain injuries [15]. It is also been shown that, IL-6 triggers the NGF production after TBI [8]. The injection of progesterone increases the level of NGF, which is associated with the beneficial effects of NGF in adults after TBI, which include; suppression of neuronal death process, increase of axonal growth, and inhabitation of neuronal apoptosis. It is being suggested that, the levels of NGF and IL-6 alone are not the suitable indicators for prediction of post-TBI outcomes. However, the ratio of NGF/IL-6 is important, as this ratio has a significant but negative correlation with Glasgow outcome scores (GOS) and Glasgow coma scores (GCS). This ratio was also significantly lower in the survivors compared to the non-survivors [16].
Considering the results of our previous research, which showed that progesterone injection at 0.5 h post TBI has beneficial effects on the reduction of brain edema and brain permeability [17] and also, since patients usually arrive at emergency department with a delay, in present study, the injection was administered 2 h after TBI. In addition, as mentioned above, the ratio of NGF/IL-6 has a correlation with post-TBI outcomes, in this study we investigated the therapeutic effects of progesterone after TBI on ovariectomized rats by measuring these two cytokines and determining the ratio of NGF/IL-6.
2. Materials and method
2.1. Animals
In this study, female Wistar rats (weighing from 200 to 250 g, and 5–7 months of age) were purchased from Animal Center of Kerman University of Medical Sciences, Iran. The rats were kept in closed cages in groups (Width 30 cm and length 60 cm). In addition, we did not have environmental enrichment, animal facility status, and specific pathogen free (SPF) in the cages. This study was approved by the Ethical Committee (No: EC/KNRC/95-22) of Kerman University of Medical Sciences and in accordance with internationally approved principles for animal use and care, as found in US guidelines (NIH publication#85-23, revised in 1985). The rats were kept at 20–22 °C and humidity (50%) in controlled conditions with a 12 h light/dark cycle. They also had free access to food and water in the animal house of Kerman Faculty of Medicine.
2.2. Method of bilateral ovariectomy
The female rats were anesthetized by 60 mg/kg thiopental (i.p) injection. The subabdominal area was shaved and a 2-cm incision was made. The skin, fascia and abdominal muscles were opened and the fats and intestine were sheared off until the uterus and its tubes were visible. Catgut 0–4 thread was then wrapped around the uterus tube and vascular base of the ovaries in the proximal area. We poured 1–2 ml of saline in the abdomen and then muscles and skin were pulled back and were stitched by catgut and 0–2 silk thread. The wound was washed with betadine solution. All experimental rats were ovariectomized (OVX) two weeks before the experiment [18].
2.3. Experimental groups
The animals were divided into 7 groups with 7 rats in each group. The groups were as follows: 1) Sham group Sh): OVX rats received no vehicle or drug. 2) TBI group: OVX rats that received brain trauma. 3) Vehicle-treated group (Veh): OVX rats that received progesterone vehicle (sesame oil) after the TBI [19]. 4) LP-treated group (LP): OVX rats that received low dose of progesterone (1.7 mg/kg, Aburaihan Pharmaceutical Co., Iran) [17]. 5) HP-treated group (HP): OVX rats that received high dose of progesterone (8 mg/kg) [17]. 6) CP group (CP): Six h after TBI, the rates received two 20mm Silastic capsules (1.55mm i.d., 3.18mm o.d, Dow Corning Corp., Midland, MI), each containing 30mm of crystalline progesterone [20, 21]. The CP level corresponds to about 10–20 ng/ml that is equivalent to basal levels of progesterone in the estrous cycle [22]. 7) Cveh group (Cveh): OVX rats were injected with vehicle (sesame oil) capsules at 6 h after trauma [23]. In LP and HP groups, drugs were administered intraperitoneally (i.p) 2 h after a diffuse TBI, which was induced by Marmarou's method [19, 23]. Serum and CSF levels of IL-6 or NGF were measured in all groups after 48 h [24], except CP or Cveh groups, which were measured 1 week [25] after the TBI.
2.4. Preparation and implantation of silastic capsules
Progesterone and vehicle capsules were prepared by cutting silastic brand silicon tubing (Dow Corning Corp, Midland, MI). Progesterone was suspended in sesame oil (30mm in length, inner/outer diameter: 1.575/3.175mm) 10–20 ng/ml, plugged with five mm of wooden applicator sticks and sealed with silastic adhesive. The capsules were soaked in either sesame oil or the same concentration of hormone. Immediately before use, the capsules were rinsed by 95% ethanol and then washed with saline (Deurveilher et al., 2008). To administer the capsules, all animals were anesthetized with 60 mg/kg thiopental (i.p), then the loose skin below their neck was cut, and a tunnel was dissected caudally for capsule implantation. The skin was subsequently sutured [6]. Since the capsule's content was being released slowly and gradually, the parameters were measured in CP and Cveh groups one week after the capsule implementation [21].
2.5. Induction of diffuse traumatic brain injury (TBI)
Diffuse TBI was induced in the rates by a device made in Physiology Department of Kerman University of Medical Sciences according to Marmarou's method. Before inducing the TBI, all rates were anesthetized (with thiopental, 60 mg/kg, i.p) and intubated. For induction of TBI, the skin of the rat's skull was covered with a steel disk (10mm in diameter and 3 mm tick) and a 250 g weight was dropped on the disk from a 2-meter height. After the induction of diffuse TBI, the rats were immediately connected to the animal respiratory pump (TSA respiratory compact, Bad Homburg, Germany). After spontaneous breathing, the rats were detached from the ventilator and returned to their cage [26].
2.6. Determination of brain edema
The brain edema of each rat was assessed by measuring brain water content (BWC) at 48 h [27] and 7 days [28] following the diffuse TBI. The rats were anesthetized and the brain was removed and weighed (wet tissue weight). The brains were incubated at 100 inside an autoclave for 48 h (Memmert, Germany) and then were reweighed (dry weight). The percentage of water in each rat was calculated by the following formula: BWC (%) = [(wet tissue weight-dry tissue weight)/wet tissue weight] ∗100 [27].
2.7. (VCS) Veterinary coma scale
The neurological outcomes were measured according to the VCS and were expressed as a score ranging from 3 to 15, which were the sum of three functions; motor function (score range 1–8), eye function (score range 1–4), and respiration function (score range 1–3). According to VCS criteria, higher scores indicate better neurological outcomes, and lower scores indicate worse neurological outcomes. In the present study, the outcomes were measured 1 h before and immediately after the TBI (Time 0). The measurements were continued at 1, 4, and 24 h after the [29].
2.8. Measurement of IL-6 and NGF in the serum and CSF
A: In order to extract IL-6 and NGF from CSF, the animal was placed in the stereotaxic machine after thiopental anesthesia and its head was tilted horizontally 30–40°. The end of polyethylene tube (PE10) was then inserted slowly into the cisterna magna space and the CSF was collected [30].
B: To measure the IL-6 and NGF in the serum, blood samples were collected from the heart and then they were centrifuged (Hrraeus, Germany) at 3,000 rpm for 15 min. Then, the serum was collected in micro tube (coagulant tubes) [31] and analyzed for IL-6 and NGF by ELISA using commercial kits (IL-6, catalogue number: ESB2701082 from the Eastbiopharm, USA; NGF, catalogue number: EKU06179 from the Zell bio, Germany).
2.9. Statistical analysis
Quantitative data are presented as mean ± SEM. The Shapiro test was used to check the normal distribution of data. Variables in the experimental groups were compared by one-way ANOVA or independent t-test. The comparison of CSF and serum levels of NGF and IL-6 were analyzed by independent t-test. The Tukey LSD test was used for the ANOVA post-hoc analysis. Percentage of changes in the rats of each group was calculated by the equation: 100 percent × [(data of hormone group − data of vehicle group)/data of vehicle group]. Software SPSS-20 was used in the statistical analysis and P-values of less than 0.05 were considered to be statistically significant.
3. Results
3.1. Brain edema
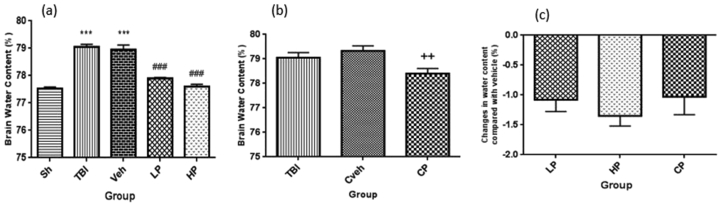
Changes in BWC in the ovariectomized rats are shown in Figure 1. The amount of BWC in TBI (79.06% ± 0.09) and vehicle (78.95% ± 0.17) groups significantly increased compared to sham group (77.52% ± 0.06) (P < 0.001). After LP and HP injections, the amount of BWC significantly reduced to 77.9% ± 0.03 and 77.6% ± 0.08, respectively compared to the vehicle group (P < 0.001) (Figure 1a). The BWC increase was significantly attenuated in CP–treated group (78.4% ± 0.21) compared to Cveh group (79.33% ± 0.2, P < 0.01) (Figure 1b). Percentage changes in BWC of different progesterone-treated rats in comparison with the vehicle rats are shown in Figure 1c. There were no significant percentage changes of BWC in the treated groups.
Figure 1.
(a) The effect of progesterone administration at 2 h after TBI on brain water content (%) in OVX rats (n = 7). ∗∗∗P < 0.001, vs. Sh. ###P < 0.001, vs. Veh. (b) The effect of capsule form of progesterone on brain water content (%) in OVX rats (n = 7). ++P < 0.01, vs. Cveh (c). Changes in percentage of water content after TBI groups compared to vehicle group in rats treated with progesterone (n = 7). No significant difference was observed between LP, HP or CP groups. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
3.2. CSF level of NGF
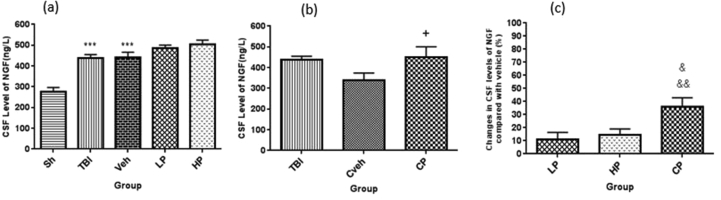
The CSF level of NGF was significantly higher in OVX rats with TBI (438.09 ± 16.52 ng/L) and vehicle groups (442.45 ± 23.21 ng/L) compared to sham group (276.95 ± 19.52 ng/L1) (P < 0.001). However, the CSF level of NGF in the LP (486.90 ± 13.52 ng/L) and HP (503.9 ± 20.23 ng/L) groups was not significantly different from the vehicle group (Figure 2a). The amount of NGF in CSF in OVX rats was different between CP (449.24 ± 51.2 ng/L) and Cveh (339.64 ± 33.99 ng/L) groups (P < 0.05) (Figure 2b). The percentage changes in CSF level of NGF in different progesterone-treated rats and the vehicle rats are shown in Figure 2c. The percentage changes in NGF level in the CP-treated group (36% ± 6.98) was significantly higher than the LP (11.15% ± 5.26) and HP (14.33% ± 4.69)-treated groups (P < 0.01 and P < 0.05, respectively).
Figure 2.
(a) The effect of progesterone administration at 2 h after TBI on CSF levels of NGF (ng/L) in OVX rats (n = 7). ∗∗∗P < 0.001, vs. Sh. (b) The effect of capsule form of progesterone on CSF levels of NGF (ng/L) in OVX rats (n = 7). + P < 0.05, vs. Cveh. (c) Changes in percentage of CSF of NGF after TBI compared with vehicle in rats treated with progesterone (n = 7). &&P < 0.01, vs.LP. & P < 0.05, vs. HP. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
3.3. CSF level of IL-6
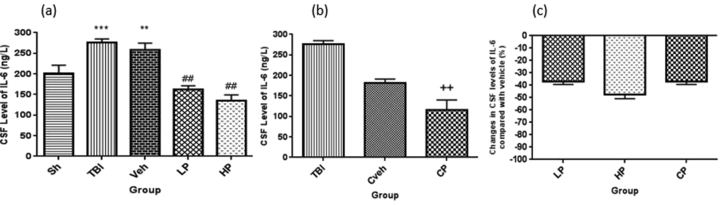
The CSF level of IL-6 was significantly higher in OVX rats with TBI (275.55 ± 9.1 ng/L) and vehicle (258 ± 16.51 ng/L) rats than sham rats (200.98 ± 19.95 ng/L) (P < 0.001 and P < 0.01, respectively). In addition, the amount of IL-6 in CSF was lower in the LP (161.98 ± 9.1 ng/L) and HP (135.72 ± 13.1 ng/L) groups than the vehicle group (P < 0.01) (Figure 3a.). The CSF level of IL-6 was significantly lower in the CP (116.19 ± 24) in comparison with the Cveh group (180.96 ± 10.28, P < 0.001) (Figure 3b). The percentage changes in serum level of IL-6 in all progesterone-treated rats compared to vehicle rats are shown in Figure 3c. We observed no significant difference in the percentage changes of CSF level of IL-6 in the groups.
Figure 3.
(a) The effect of progesterone administration at 2 h after TBI on CSF levels of IL-6 (ng/L) in OVX rats (n = 7). ∗∗∗P < 0.001, vs. Sh.∗∗P < 0.01, vs. Sh. ##p < 0.01, vs. Veh. (b) The effect of capsule form of progesterone on IL-6 levels of IL-6 (ng/L) in OVX rats (n = 7). ++, P < 0.01.vs Cveh. (c) Changes in percentage of CSF of IL-6 after TBI compared with vehicle in rats treated with progesterone (n = 7). No significant different between LP, HP or CP groups. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
3.4. NGF/IL-6 ratio in CSF
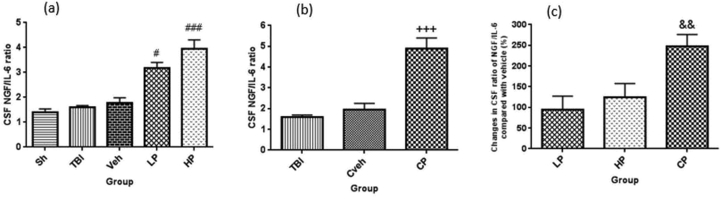
There was no significant difference in CSF ratio of NGF/IL-6 in OVX rats with TBI (1.59 ± 0.08), vehicle rates (1.77 ± 0.21), and sham (1.41 ± 0.12) groups. However, the ratio of NGF/IL-6 in the LP (3.18 ± 0.22) and HP (3.94 ± 0.37) treated rats showed a significant increase compared to the vehicle group (P < 0.05 and P < 0.001, respectively) (Figure 4a). In OVX rats, this ratio showed a significant increase (P < 0.001) in CP (4.9 ± 1.5) group in comparison to Cveh (1.94 ± 0.31) group (Figure 4b). The percentage changes in CSF ratio of NGF/IL-6 in all progesterone-treated rats compared to vehicle rats are shown in Figure 4c. The percentage changes of this ratio in CP-treated (247.27% ± 28.96) rat was higher than the LP (94.02% ± 33.17) and HP (124.52% ± 33.17)-treated rats (P < 0.01).
Figure 4.
(a) The effect of progesterone administration at 2 h after TBI on CSF ratio of NGF/IL-6 in OVX rats (n = 7). #P < 0.05, vs. Veh. ###P < 0.001, vs. Veh. (b) The effect of capsule form of progesterone on CSF ratio of NGF/IL-6 in OVX rats (n = 7). +++ P < 0.001, vs. Cveh. (c) Changes in percentage of CSF ratio of NGF/IL-6 after TBI compared with vehicle in rats treated with progesterone (n = 7). && P < 0.01, vs. LP and HP. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
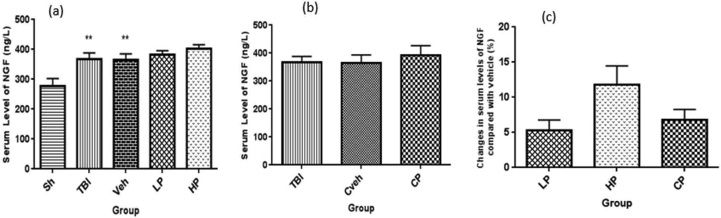
3.5. Serum level of NGF
The serum level of NGF was significantly different between OVX rats with TBI (367.39 ± 21.29 ng/L), vehicle (365.35 ± 19.96 ng/L) rates, and sham group (278.45 ± 24.42 ng/L) (P < 0.01). The amount of NGF in serum was not significantly different in the LP (383.21 ± 12.24 ng/L) and HP (403.45 ± 12.53 ng/L) groups, and there was no significant difference between the treatment groups and vehicle groups (Figure 5a). In addition, the level of NGF was not significantly different in CP (391.78 ± 35.03 ng/L) and Cveh (365.67 ± 28.45 ng/L) groups (Figure 5b). The percentage changes in serum level of NGF in all progesterone-treated rats compared to vehicle rats are shown in Figure 5c. There was no significant difference in the percentage changes of serum level of NGF in the treated groups.
Figure 5.
(a) The effect of progesterone administration at 2 h after TBI on serum levels of NGF (ng/L) in OVX rats (n = 7). ∗∗P < 0.01, vs. sh. (b) The effect of capsule form of progesterone on CSF levels of NGF (ng/L) in OVX rats (n = 7). No significant difference between TBI, CP or Cveh groups. (c) Changes in percentage of serum of NGF after TBI compared with vehicle in rats treated with progesterone (n = 7). No significant difference between LP, HP or CP groups. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
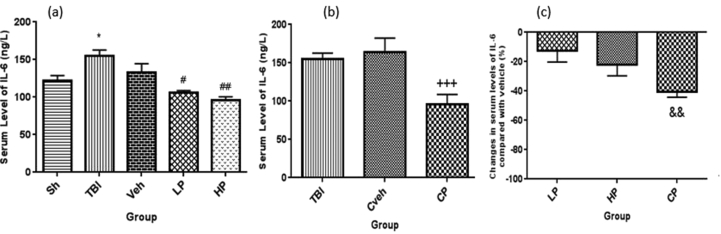
3.6. Serum levels of IL-6
The serum level of IL-6 was significantly different between OVX rats with TBI (154.96 ± 7.79 ng/L) and sham group (121.50 ± 7.30 ng/L) (P < 0.05). In addition, the serum level of IL-6 was significantly lower in the HP (95.69 ± 4.9 ng/L, P < 0.01) and LP (106.10 ng/L ±2.68) (P < 0.05) treated groups compared to the vehicle –treated (132.53 ± 12.03 ng/L) group (Figure 6a). The level of IL-6 in serum was significantly different between the CP and Cveh groups (P < 0.001) (Figure 6b). The percentage changes in serum level of IL-6 in all progesterone-treated rats compared to vehicle rats are shown in Figure 6c. The percentage changes of IL-6 were significantly higher in CP-treated (-41% ± 5) rats compared to LP -treated (-12% ± 11) rats (P < 0.01).
Figure 6.
(a) The effect of progesterone administration at 2 h after TBI on serum levels of IL-6 (ng/L) in OVX rats (n = 7). ∗P < 0.05, vs. Sh.#P < 0.05, vs. Veh. ##P < 001, vs Veh. (b) The effect of capsule form of progesterone on CSF levels of IL-6 (ng/L) in OVX rats (n = 7). +++P < 0.001, vs Cveh. (c) Changes in percentage of serum of IL-6 after TBI compared with vehicle in rats treated with progesterone (n = 7). && P < 0.01, vs LP group. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
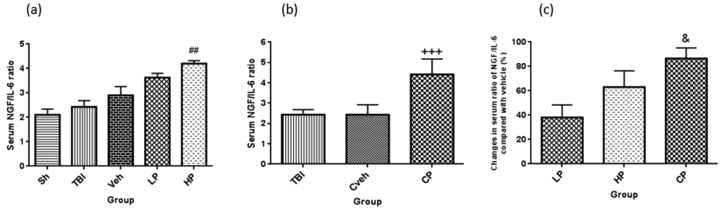
3.7. NGF/IL-6 ratio in serum
There was no significant difference in the NGF/IL-6 ratio in Serum between the OVX rats with TBI (2.43 ± 0.25), vehicle (2.93 ± 0.36) rates and Sham (2.19 ± 0.24) groups. Although high dose of progesterone could significantly increase the ratio of NGF/IL-6 (4.25 ± 0.12) compared to vehicle group (P < 0.01) (Figure 7a.). After the implantation of progesterone capsule (4.42 ± 0.75) this ratio increased compared to Cveh (2.43 ± 0.49) group (P < 0.001) (Figure 7b).The percentage changes in serum ratio of NGF/IL-6 in all progesterone-treated rats compared to vehicle rats are shown in Figure 7c. The increase in the percentage changes of this ratio was stronger in CP-treated (86.37% ± 8.83) group compared to LP -treated (37.96% ± 10.41) group (P < 0.05).
Figure 7.
(a) The effect of progesterone administration at 2 h after TBI on serum ratio of NGF/IL-6 in OVX rats (n = 7). ##P < 0.01, vs. Veh. (b) The effect of capsule form of progesterone on serum ratio of NGF/IL-6 in OVX rats (n = 7). +++P < 0.001, vs. Cveh. (c) Changes in percentage of serum ratio of NGF/IL-6 after TBI compared with vehicle in rats treated with progesterone (n = 7). & P < 0.05, vs. LP. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP, progesterone capsule; Cveh, capsule vehicle.
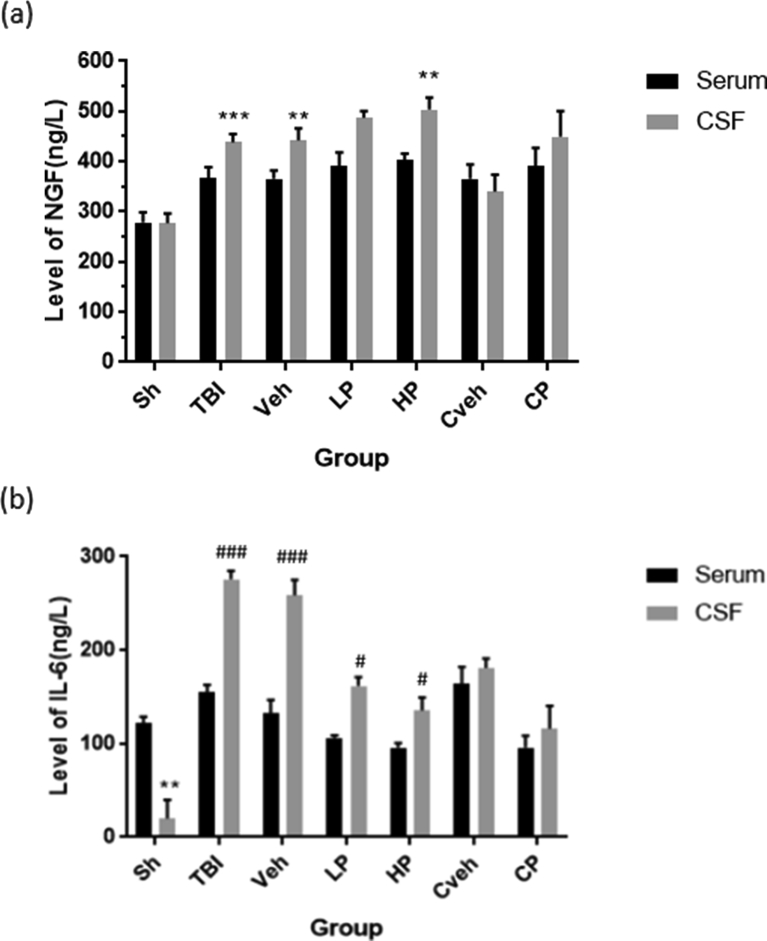
3.8. Comparison of the CSF and serum levels of NGF and IL-6
The CSF level of NGF was significantly higher than the serum NGF level in TBI, vehicle, and HP groups (P < 0.001, P < 0.01, and P < 0.01, respectively). In addition, the CSF level of IL-6 was higher than the serum IL-6 level in TBI, vehicle (P < 0.001), LP, and HP rats (P < 0.05). TBI has increased NGF in the CSF compared to serum. The difference was eliminated in the serum and CSF levels of NGF in the LP group, but it remained in the HP group. However, the CSF level of IL-6 has decreased in LP and HP groups, but it was still greater than serum levels in sham group. Also similar to IL-6, there was no difference between the levels of NGF in CSF and serum in the CP group (Figure 8).
Figure 8.
Changes in CSF and serum concentration of NGF and IL-6 in different groups following diffuse traumatic brain injury (n = 7).(a) ∗∗∗ P < 0.001 and ∗∗P < 0.01 vs serum. (b) ###P < 0.001, ##P < 0.01, and #P < 0.05 vs serum. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP,progesterone capsule; Cveh, capsule vehicle.
3.9. Neurological outcomes (VCS)
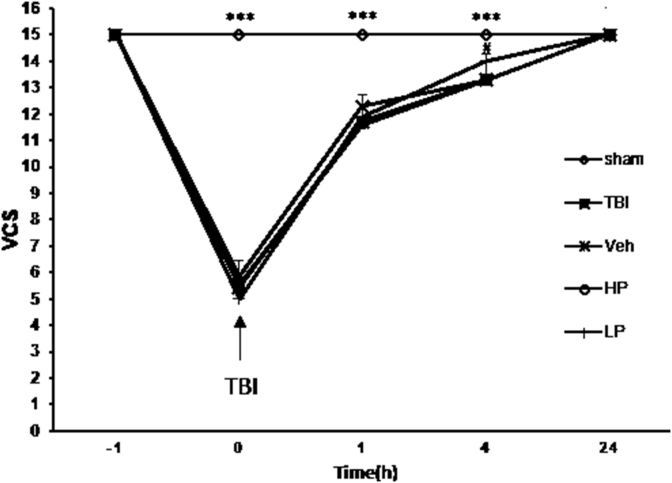
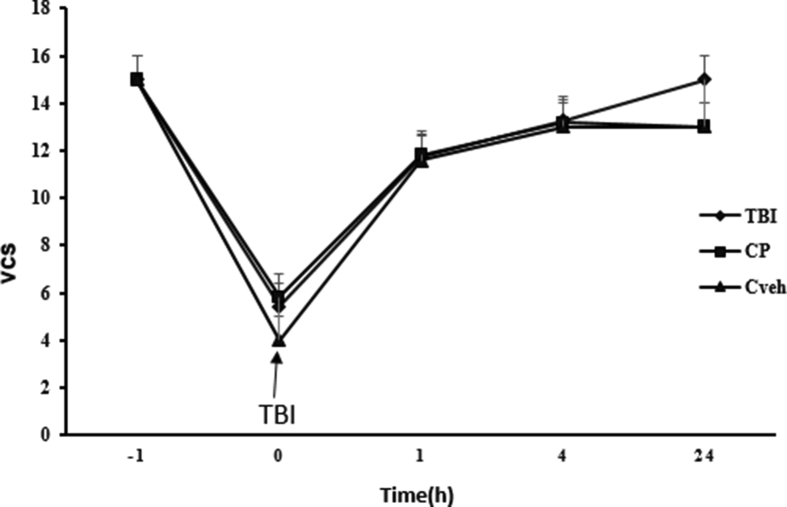
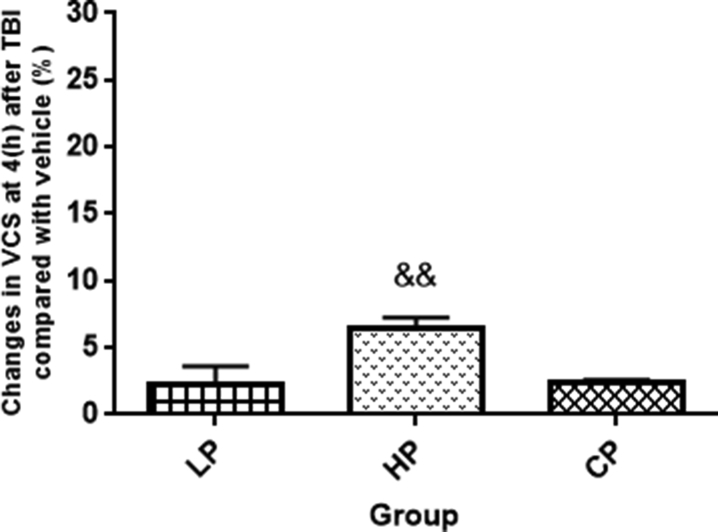
Since the maximum neurologic score was obtained in the groups within 24 h and the difference between treatment groups and the sham group disappeared, the VCS index was not measured for more than one day. Figure 9 shows VCS at different times in all groups. At immediately, 1 and 4 h after TBI, this index was lower in OVX rats with TBI (5.42 ± 0.64, 11.42 ± 0.20, and 13.28 ± 0.19 at immediately, 1 and 4 h, respectively, P < 0.001) and vehicle rates compared to sham (15 ± 0 at all the times) group (Figure 9). At 1 and 24 h after TBI, no significant difference in this index was observed between the vehicle, HP, and LP groups (Figure 9). However, 4 h after TBI, the VCS in HP (14 ± 0.21) group was significantly higher than vehicle group (Figure 9). No significant difference in this index was seen in OVX rats and CP and Cveh groups at different times (Figure 10). The percentage changes in the VCS in all progesterone-treated rats compared to vehicle rats are shown in Figure 11. The increase in this index was stronger in HP (6.51% ± 0.74)-treated group compared to the LP and CP -treated groups (P < 0.01).
Figure 9.
Changes in VCS at different times in treated and non-treated rats (n = 7). ∗∗∗P < 0.001 immediately after TBI and at 1 h and4 h after TBI for sham group vs. TBI and Veh groups. #, P < 0.05 at 4 h after TBI for HP group vs. Veh group.
Figure 10.
Changes VCS at different times in treated and non-treated rats (n = 7). No significant different between Cp and Cveh groups all the times.
Figure 11.
Changes in percentage of VCS at 4 h after TBI with vehicle in treated rats (n = 7). && P < 0.05 HP group vs. and CP groups. Sh, sham; Veh, vehicle; LP, low progesterone; HP, high progesterone; CP,progesterone capsules; Cveh, capsule vehicle.
4. Discussion
IL-6 and NGF are among important biomarkers that effect TBI outcomes. Although it has been reported that, values of these two biomarkers alone cannot be a good indicator of TBI outcome, the NGF/IL-6 ratio can still be important. In the present study, the NGF/IL-6 ratio was investigated after the administration of different forms of progesterone in OVX rats. The main findings of our study included: 1) Both low and high dose of progesterone and CP reduced the brain edema. 2) Injection of different doses of progesterone did not increase the level of NGF and only CP increased the NGF in CSF, while both injected doses, and CP reduced the level of IL- 6 in CSF after TBI, but high dose of progesterone, and CP reduced the serum IL-6. 3) Low and high doses of progesterone, and CP increased the ratio of NGF/IL-6 in CSF after TBI, but the serum level of this ratio was only increased by high dose and CP. 4) The levels of NGF and IL-6 in TBI and HP groups were higher in CSF than in serum (see Figure 8).
4.1. Effect of doses and different methods of progesterone administration on brain edema
The results of this study showed that, TBI in OVX rats caused more BWC in comparison with the sham (intact) animals, so the BWC increased by 1.54%, 1.43%, and 1.81%, in the TBI, vehicle, and Cveh groups, respectively and this increase was reduced by 1.05% in LP, 1.3% in HP, and 0.9% in CP groups. This effect was neither dependent on dose, nor on the method of administration, because there was no significant difference between the two doses and between the LP, HP, and CP groups. Many experimental studies have revealed the neuroprotective effects of progesterone [32]. Soltani et al confirmed that progesterone inhibits the brain edema. Maghool and O'Connor showed the reduction of BBB permeability in TBI by progesterone [4, 6]. In addition, Maghool et al reported that sustained release of progesterone (capsule) is beneficial in the treatment of TBI [6]. Progesterone also protects neurons by decreasing inflammatory cytokines after TBI [33].
Some of the possible mechanism(s) by which LP and HP, as well as capsule form of progesterone reduce brain edema include; decreasing the expression of AQP4 [5], reducing inflammatory cytokines such as IL-6 [34], decreasing cytokine receptors [35], decreasing the expression of MMP2 (Matrix Metallo Proteinases) and MMP9 [36], increasing the expression of caludin5 and occludin1 [37], increasing p-glycoprotein pump (PGP) expression [38], having antioxidant properties [39].
Findings of some studies on progesterone do not support the results of present study, including decrease in brain edema after TBI [11] and increased cell death and worsening of brain inflammation [40]. Possible reasons for this differences could be the type of study (animal or human), animal's sex, animal's species, duration of drug administration and method of brain edema induction as well as types of brain injuries [40].
4.2. The effect of progesterone on the NGF level in serum and CSF
The other part of this study showed that, the CSF level of NGF in OVX rats increased in TBI, vehicle, and Cveh groups by 58.18%, 65.5%, and 22.6%, respectively and in serum by 31.94%, 31.2%, and 32.31% respectively. Among different administration forms of progesterone, only CP could increase the level of NGF in CSF after TBI. Comparing the level of NGF in CSF and serum showed that, the NGF level was higher in CSF than in serum. This means that, perhaps measuring the level of this biomarker in the CSF is better for detecting changes than measuring its level in the serum. The higher NGF level in CSF suggests that, the brain's injured tissue is the source of this biomarker. These results also indicated that, perhaps the progesterone form or the duration of administration contributed to the progesterone's neuroprotective effects, because it was effective in the CP group as subcutaneous capsule when administered for a longer time.
Consistent with the above results, it has been reported that NGF increases in serum and CSF of TBI patients, and this increase is significantly more in CSF then the serum [41]. It has also been shown that, the increase in NGF in traumatized children is consistent with appropriate neurological outcomes, and it is an essential element for repairing damaged cells caused by TBI [42]. The NGF levels are also elevated in ischemia [43]. This peptide has been reported to cause neuronal proliferation, differentiation and synaptogenesis, improve motor and cognitive function, and decrease brain edema [44].
In agreement with the current study, progesterone has been reported to increase NGF production after TBI and thereby decreases apoptosis and improves motor function following TBI [45]. It has also been shown that, progesterone increases the expression of NGF in peripheral organs, which plays an important role in regulating neuronal plasticity, nociception, and restoration of sympathetic nervous system [46]. The mechanism for beneficial effects of progesterone is that, it increases NGF by stimulating the expression and regulation of NGF receptors [45]. In addition, progesterone has the capacity to increase the NGF mRNA content, translation of the NGF gene or stability of NGF protein [46]. It is also possible that progesterone exerted elects on NGF via glucocorticoid receptors. Glucocorticoids stimulate increases the expression of NGF, in the brain [47].
The possible mechanism (s) of neuroprotective effect of NGF after brain injury include: reduction of intracellular calcium [48] and promotion of antioxidant activity [49].
The results of present study are not consistent with the findings of some studies that showed NGF reduction by progesterone following brain injury [50] and lack of NGF effect on neuronal recovery following TBI [51]. Possible differences in the results of the present study with others can be due to the difference in the type of brain injuries [50], the dosage and method of progesterone administration, and the severity of neurological injury [51].
4.3. Effect of progesterone on the IL-6 level in serum and CSF
In another section, the results of this study showed that, IL-6 level in the TBI group increased by 37.1% in CSF and 53.27% in serum 48 h after the TBI. The IL-6 levels in CSF also reduced by 37.09% in LP, 47.49% in HP, and 37.80% in CP. The IL-6 levels in serum also reduced by 13% in LP, 27.79% in HP, and 41.57% in CP using low and high doses as well as capsule form of progesterone, respectively. Since there was no significant difference in serum level of IL-6 between the vehicle and sham groups, it is likely that the vehicle group had inhibitory effects on this cytokine. In all treatment groups except the CP group, the level of this biomarker was greater in CSF than serum, which could be due to two reasons: First, it is likely that there was a greater increase in CSF after TBI, so despite the decreasing effects of administered doses, the level of IL-6 in CSF was higher than the serum. Second, the CP group, in comparison with the injection groups, caused a similar reduction in serum and CSF. The elevated level of IL-6 in the CSF compared to serum suggested that, the brain's injured tissue was the source of cytokine production. An increase of a biomarker in CSF is a more reliable indicator than in serum, because it excludes the increases of poly-traumas. Furthermore, the effect was dependent on the method of administration, because the CP group reduced the level of this cytokine more than the HP-LP groups.
In regard to the importance of IL-6 in trauma, there are conflicting results, especially in the role of IL-6 as a predicting factor for ICP, mortality, and post-traumatic outcomes [18]. It has been reported that, the initial increase of IL-6 can have a protective role, while its delayed increase can be harmful. An increase in this cytokine in patients' CSF or serum following TBI has also been reported. There are conflicting reports about the time and the peak of IL-6 elevation, which ranges from 12 h [52] or 48 h in some studies [52], to 1–2 days [53], and 4 days after injury in other studies [54]. It seems that, the timing of cytokines' expression has a correlation with the extent of injury.
Findings of some studies are in line with the present study, including progesterone injection causes a significant decrease in IL-6 serum levels [38]. Sarkaki et al also found that a high dose of progesterone 6 h after TBI reduces IL-6 level, while its low dose causes the same decrease in 24 h [34]. Shahrokhi et al reported that not only progesterone, but also its combined form with estrogen reduces the IL-6 level [18]. Progesterone reduces ICP by decreasing IL-6 level [17]. Considering the above arguments, it seems that one of the mechanisms, by which this steroid is beneficial in improving post-TBI outcomes, is to decrease the level of IL-6. Chen et al reported that the main mechanism of progesterone in reducing inflammation after TBI is inhibition of IL-6 and IL-1β [55]. The possible mechanism(s) by which IL-6 contributes to the prolonged damage after TBI include; increasing CPP [56], the loss of neurons, impaired BBB function, and vessels' deficiency with major neurological disorders [57]. Mechanism mediating the beneficial effects of progesterone may be its anti-inflammatory properties, which is supported by our previous work that showed progesterone inhibited IL-6 production after TBI [32, 58]. Probably mononuclear cells and astrocyte secretion of IL-6 in response to TBI is blocked by progesterone treatment [59, 60, 61]. Toll-like receptor 4 (TLR4) activates pro-inflammatory pathways including inflammatory cytokines, such as IL-6 and TNF-α. It has been reported that, there are cross talk between TLR4 and progesterone receptor, and this mechanism may be responsible for progesterone mediated inhibition of the IL-6 induction [62].
There are also studies that do not confirm the results of present study, including the study of Holmin et al which found that, IL-6 level does not increase after brain injury [54]. Possible reasons for this difference can be the use of human samples [54] and the method of brain injury induction [8].
4.4. Changes in NGF/IL-6 ratio in CSF and serum in OVX rats
The ratio of these two biomarkers was also measured in the present study. This ratio in CSF increased by LP 94.02%, HP 124.52%, and CP 247.27%. It also increased in serum by 62.94% and 86.37% respectively, using high dosage and capsule form of progesterone. Although no significant difference was observed between the two administered doses, the role of consumption method was obvious, as the CP group increased this ratio in the CSF 153.25% and 12.75%, more than the LP and HP groups, respectively.
It seems that, the reason for the lack of change in NGF/IL-6 ratio after TBI was the alignment change in the levels of NGF and IL-6, so that they both increased after TBI. On the other hand, NGF level was also higher than IL-6 level in the treatment groups. It was found that, although all administration doses and methods reduced the level of IL-6, the HP-induced reduction in this cytokine was higher than other groups, and this may be due to the higher proportion of this ratio in HP compared to LP. However, examining the NGF revealed that only CP increased the NGF. Therefore, it can be concluded that, this high ratio in the CP group compared to other groups was due to a higher increase in NGF and not a further reduction in IL-6. So, it can be said that, in cases where we need to increase NGF, the capsule method can be used, and in cases where reduction in IL-6 is required, injection methods should be recommended.
It has been reported that, NGF increases and IL-6 decreases over time [42]. Many cytokines, especially interleukins, have neurotrophic effects and produce certain neurotrophic factors. Also, IL-6 has been reported to stimulate the production of NGF via the ERK pathway [41] and under various conditions [8].
It has been argued that, the levels of NGF and IL-6 alone are not good indicators for predicting post-TBI outcomes, as there are many conflicting studies some of which show their advantages [16] and others show their disadvantages [42]. It has been reported that, the NGF/IL-6 ratio is correlated with GCS and GOS scores [16, 42]. Our study also found that, the measurement of ratio is a better indicator for evaluating the effects of progesterone after TBI, because despite the lack of changes in NGF after injection of progesterone doses, the ratio of NGF/IL-6 increased by both doses. The results of our study are not consistent with the results of other studies [16, 42], which have reported the decrease of this ratio with the improvement of neurological outcomes, such as improved GCS and GOS scores. The difference in the results of these studies could be due to differences in the study sample that was patients and the measurement index and time.
4.5. The effect of progesterone on VCS
The results of neurological score (motor, ocular, and respiratory) measured in this study in OVX rates showed that, a higher VCS score was obtained at 4 h after TBI for high doses of progesterone. This means that, this dose was more effective at 4 h after TBI than other doses. Also, although all treatment groups had higher scores compared to vehicle at this time, this difference was not significant. The results of this study are in agreement with the findings of Dehghan et al [63], and Zahedi Asl et al [64] who showed that VCS decreases immediately after brain injury. Since the gradual release of progesterone was not effective in increasing VCS at any time in this study, this method is not recommended in any study that was to consider the increase in VCS. The mechanism by which progesterone increases VCS may be due to its beneficial effect on reducing brain edema and blood-brain barrier permeability, as shown in our study. Stien et al reported that this sex hormone may increase neurological scoring after TBI by reducing ICP [18]. Shahrokhi et al have also confirmed this finding. In addition, increased CBF, which is directly related to ICP, leads to increased VCS [65]. However, Shahrokhi et al showed that at 24 h post-brain injury, there was an increase in VCS of the progesterone-treated group, which is contrary to the results of our present study [18]. This could be due to the differences in time and type of anesthetic drug used in these studies.
5. Conclusion
The current study suggests that, progesterone has a neuroprotective effect on OVX rats after TBI by reducing IL-6 or increasing NGF. However, the increase in NGF occurred only when progesterone was administered as capsule over a long period of time. On the other hand, although, the NGF/IL-6 CSF ratio increased by all forms of progesterone administration, it increased more by capsule compared to injection forms. Furthermore, an increase in the level of NGF and IL-6 was higher in CSF than in serum after TBI and the effects of high dose of progesterone on the increase of NGF or the reduction of IL-6 was higher in CSF than in serum. It seems that, the brain is the source of NGF and IL-6 production, and the neuroprotective effects of progesterone are due to the changes in the production of these two biomarkers in the brain. Although the ratio of NGF/IL-6 in CSF increased by both progesterone dosages, this ratio only increased in the serum by a high dose of progesterone. In addition, in all cases the effect of capsule form was probably greater than both injection forms. So, in future studies it is suggested that, instead of measuring these biomarkers individually, their ratio should be determined as a variable, to identify the neuroprotective effect of a drug. In conclusion, the effect of progesterone was greater in CP group than injection groups, but further studies are needed to confirm this.
Declarations
Author contribution statement
Shirazpour Sara: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Khaksari Mohammad: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Shahrokhi Nader, Salmani Neda: Analyzed and interpreted the data.
Iranpour Maryam, Shahryari Marzieh, Jafari Elham: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by Kerman University of Medical Sciences.
Competing interest statement
The authors no declare conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would like to thank the manager of Kerman Neuroscience Center.
References
- 1.Dewan M.C., Rattani A., Gupta S., Baticulon R.E., Hung Y.-C., Punchak M., Agrawal A., Adeleye A.O., Shrime M.G., Rubiano A.M. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018;130(4):1080–1097. doi: 10.3171/2017.10.JNS17352. [DOI] [PubMed] [Google Scholar]
- 2.Iaccarino C., Carretta A., Nicolosi F., Morselli C. Epidemiology of severe traumatic brain injury. J. Neurosurg. Sci. 2018;62(5):535–541. doi: 10.23736/S0390-5616.18.04532-0. [DOI] [PubMed] [Google Scholar]
- 3.Wilson L., Stewart W., Dams-O'Connor K., Diaz-Arrastia R., Horton L., Menon D.K., Polinder S. The chronic and evolving neurological consequences of traumatic brain injury. Lancet Neurol. 2017;16(10):813–825. doi: 10.1016/S1474-4422(17)30279-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Connor C.A., Cernak I., Vink R. Both estrogen and progesterone attenuate edema formation following diffuse traumatic brain injury in rats. Brain Res. 2005;1062(1-2):171–174. doi: 10.1016/j.brainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 5.Guo Q., Sayeed I., Baronne L.M., Hoffman S.W., Guennoun R., Stein D.G. Progesterone administration modulates AQP4 expression and edema after traumatic brain injury in male rats. Exp. Neurol. 2006;198(2):469–478. doi: 10.1016/j.expneurol.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Maghool F., Khaksari M. Differences in brain edema and intracranial pressure following traumatic brain injury across the estrous cycle: involvement of female sex steroid hormones. Brain Res. 2013;1497:61–72. doi: 10.1016/j.brainres.2012.12.014. [DOI] [PubMed] [Google Scholar]
- 7.Shahrokhi N., Soltani Z., Khaksari M., Karamouzian S., Mofid B., Asadikaram G. The serum changes of neuron-specific enolase and intercellular adhesion molecule-1 in patients with diffuse axonal injury following progesterone administration: a randomized clinical trial. Arch. Trauma Res. 2016;5(3) doi: 10.5812/atr.37005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kossmann T., Hans V., Imhof H.-G., Trentz O., Morganti-Kossmann M.C. Interleukin-6 released in human cerebrospinal fluid following traumatic brain injury may trigger nerve growth factor production in astrocytes. Brain Res. 1996;713(1-2):143–152. doi: 10.1016/0006-8993(95)01501-9. [DOI] [PubMed] [Google Scholar]
- 9.Abbas A.K., Lichtman A.H., Pillai S. Elsevier Health Sciences; 1994. Cellular and Molecular Immunology. [Google Scholar]
- 10.Belelli D., Lambert J.J. Neurosteroids: endogenous regulators of the GABA A receptor. Nat. Rev. Neurosci. 2005;6(7):565–575. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 11.Jones N.C., Constantin D., Prior M.J., Morris P.G., Marsden C.A., Murphy S. The neuroprotective effect of progesterone after traumatic brain injury in male mice is independent of both the inflammatory response and growth factor expression. Eur. J. Neurosci. 2005;21(6):1547–1554. doi: 10.1111/j.1460-9568.2005.03995.x. [DOI] [PubMed] [Google Scholar]
- 12.Freeman R.S., Burch R.L., Crowder R.J., Lomb D.J., Schoell M.C., Straub J.A., Xie L. NGF deprivation-induced gene expression: after ten years, where do we stand? Prog. Brain Res. 2004;146:111–126. doi: 10.1016/S0079-6123(03)46008-1. [DOI] [PubMed] [Google Scholar]
- 13.Kawaja M.D., Gage F.H. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron. 1991;7(6):1019–1030. doi: 10.1016/0896-6273(91)90346-2. [DOI] [PubMed] [Google Scholar]
- 14.Kossmann T., Stahel P.F., Lenzlinger P.M., Redl H., Dubs R.W., Trentz O., Schlag G., Morganti-Kossmann M.C. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood–brain barrier dysfunction and nerve growth factor production. J. Cerebr. Blood Flow Metabol. 1997;17(3):280–289. doi: 10.1097/00004647-199703000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Gadient R., Cron K., Otten U. Interleukin-1 β and tumor necrosis factor-α synergistically stimulate nerve growth factor (NGF) release from cultured rat astrocytes. Neurosci. Lett. 1990;117(3):335–340. doi: 10.1016/0304-3940(90)90687-5. [DOI] [PubMed] [Google Scholar]
- 16.Winter C.D., Pringle A.K., Clough G.F., Church M.K. Raised parenchymal interleukin-6 levels correlate with improved outcome after traumatic brain injury. Brain. 2004;127(2):315–320. doi: 10.1093/brain/awh039. [DOI] [PubMed] [Google Scholar]
- 17.Khaksari M., Soltani Z., Shahrokhi N., Moshtaghi G., Asadikaram G. The role of estrogen and progesterone, administered alone and in combination, in modulating cytokine concentration following traumatic brain injury. Can. J. Physiol. Pharmacol. 2011;89(1):31–40. doi: 10.1139/y10-103. [DOI] [PubMed] [Google Scholar]
- 18.Shahrokhi N., Khaksari M., Soltani Z., Mahmoodi M., Nakhaee N. Effect of sex steroid hormones on brain edema, intracranial pressure, and neurologic outcomes after traumatic brain injury. Can. J. Physiol. Pharmacol. 2010;88(4):414–421. doi: 10.1139/y09-126. [DOI] [PubMed] [Google Scholar]
- 19.Feeser V.R., Loria R.M. Modulation of traumatic brain injury using progesterone and the role of glial cells on its neuroprotective actions. J. Neuroimmunol. 2011;237(1-2):4–12. doi: 10.1016/j.jneuroim.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Depaolo L.V., Barraclough C.A. Dose dependent effects of progesterone on the facilitation and inhibition of spontaneous gonadotropin surges in estrogen treated ovariectomized rats. Biol. Reprod. 1979;21(4):1015–1023. doi: 10.1095/biolreprod21.4.1015. [DOI] [PubMed] [Google Scholar]
- 21.Depaolo L.V., Rowlands K.L. Deceleration of age-associated changes in the preovulatory but not secondary follicle-stimulating hormone surge by progesterone. Biol. Reprod. 1986;35(2):320–326. doi: 10.1095/biolreprod35.2.320. [DOI] [PubMed] [Google Scholar]
- 22.Smith M.S., Freeman M., Neill J. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96(1):219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- 23.Roof R.L., Duvdevani R., Braswell L., Stein D.G. Progesterone facilitates cognitive recovery and reduces secondary neuronal loss caused by cortical contusion injury in male rats. Exp. Neurol. 1994;129(1):64–69. doi: 10.1006/exnr.1994.1147. [DOI] [PubMed] [Google Scholar]
- 24.Farbood Y., Sarkaki A., Dianat M., Khodadadi A., Haddad M.K., Mashhadizadeh S. Ellagic acid prevents cognitive and hippocampal long-term potentiation deficits and brain inflammation in rat with traumatic brain injury. Life Sci. 2015;124:120–127. doi: 10.1016/j.lfs.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 25.Hang C.-H., Shi J.-X., Li J.-S., Li W.-Q., Wu W. Expressions of intestinal NF-κB, TNF-α, and IL-6 following traumatic brain injury in rats. J. Surg. Res. 2005;123(2):188–193. doi: 10.1016/j.jss.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 26.Hajmohammadi M., Khaksari M., Soltani Z., Shahrokhi N., Najafipour H., Abbasi R. The effect of Candesartan alone and its combination with estrogen on post-traumatic brain injury outcomes in female rats. Front. Neurosci. 2019;13 doi: 10.3389/fnins.2019.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soltani Z., Khaksari M., Jafari E., Iranpour M., Shahrokhi N. Is genistein neuroprotective in traumatic brain injury? Physiol. Behav. 2015;152:26–31. doi: 10.1016/j.physbeh.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 28.Roof R.L., Duvdevani R., Heyburn J.W., Stein D.G. Progesterone rapidly decreases brain edema: treatment delayed up to 24 hours is still effective. Exp. Neurol. 1996;138(2):246–251. doi: 10.1006/exnr.1996.0063. [DOI] [PubMed] [Google Scholar]
- 29.Soltani N., Soltani Z., Khaksari M., Ebrahimi G., Hajmohammmadi M., Iranpour M. The changes of brain edema and neurological outcome, and the probable mechanisms in diffuse traumatic brain injury induced in rats with the history of exercise. Cell. Mol. Neurobiol. 2019:1–13. doi: 10.1007/s10571-019-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chyatte D. Prevention of chronic cerebral vasospasm in dogs with ibuprofen and high-dose methylprednisolone. Stroke. 1989;20(8):1021–1026. doi: 10.1161/01.str.20.8.1021. [DOI] [PubMed] [Google Scholar]
- 31.Joukar S., Najafipour H., Malekpour-Afshar R., Mirzaeipour F., Nasri H.R. The effect of passive opium smoking on cardiovascular indices of rabbits with normal and ischemic hearts. Open Cardiovasc. Med. J. 2010;4:1. doi: 10.2174/1874192401004010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khaksari M., Keshavarzi Z., Gholamhoseinian A., Bibak B. The effect of female sexual hormones on the intestinal and serum cytokine response after traumatic brain injury: different roles for estrogen receptor subtypes. Can. J. Physiol. Pharmacol. 2013;91(9):700–707. doi: 10.1139/cjpp-2012-0359. [DOI] [PubMed] [Google Scholar]
- 33.VanLandingham J.W., Cutler S.M., Virmani S., Hoffman S.W., Covey D.F., Krishnan K., Hammes S.R., Jamnongjit M., Stein D.G. The enantiomer of progesterone acts as a molecular neuroprotectant after traumatic brain injury. Neuropharmacology. 2006;51(6):1078–1085. doi: 10.1016/j.neuropharm.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 34.Sarkaki A.R., Khaksari Haddad M., Soltani Z., Shahrokhi N., Mahmoodi M. Time-and dose-dependent neuroprotective effects of sex steroid hormones on inflammatory cytokines after a traumatic brain injury. J. Neurotrauma. 2013;30(1):47–54. doi: 10.1089/neu.2010.1686. [DOI] [PubMed] [Google Scholar]
- 35.Wise P.M., Dubal D.B., Wilson M.E., Rau S.W., Böttner M. Minireview: neuroprotective effects of estrogen—new insights into mechanisms of action. Endocrinology. 2001;142(3):969–973. doi: 10.1210/endo.142.3.8033. [DOI] [PubMed] [Google Scholar]
- 36.Ishrat T., Sayeed I., Atif F., Hua F., Stein D.G. Progesterone and allopregnanolone attenuate blood–brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp. Neurol. 2010;226(1):183–190. doi: 10.1016/j.expneurol.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang C., Wang J., Li X., Liu C., Chen N., Hao Y. Progesterone exerts neuroprotective effects by inhibiting inflammatory response after stroke. Inflamm. Res. 2009;58(9):619–624. doi: 10.1007/s00011-009-0032-8. [DOI] [PubMed] [Google Scholar]
- 38.Cutler S.M., Cekic M., Miller D.M., Wali B., VanLandingham J.W., Stein D.G. Progesterone improves acute recovery after traumatic brain injury in the aged rat. J. Neurotrauma. 2007;24(9):1475–1486. doi: 10.1089/neu.2007.0294. [DOI] [PubMed] [Google Scholar]
- 39.Liu R., Wen Y., Perez E., Wang X., Day A.L., Simpkins J.W., Yang S.-H. 17β-Estradiol attenuates blood–brain barrier disruption induced by cerebral ischemia–reperfusion injury in female rats. Brain Res. 2005;1060(1-2):55–61. doi: 10.1016/j.brainres.2005.08.048. [DOI] [PubMed] [Google Scholar]
- 40.Koski C.L., Hila S., Hoffman G.E. Regulation of cytokine-induced neuron death by ovarian hormones: involvement of antiapoptotic protein expression and c-JUN N-terminal kinase-mediated proapoptotic signaling. Endocrinology. 2004;145(1):95–103. doi: 10.1210/en.2003-0803. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y.Y., Bradshaw R.A. Induction of neurite outgrowth by interleukin-6 is accompanied by activation of Stat3 signaling pathway in a variant PC12 cell (E2) line. J. Biol. Chem. 1996;271(22):13023–13032. doi: 10.1074/jbc.271.22.13023. [DOI] [PubMed] [Google Scholar]
- 42.Chiaretti A., Antonelli A., Mastrangelo A., Pezzotti P., Tortorolo L., Tosi F., Genovese O. Interleukin-6 and nerve growth factor upregulation correlates with improved outcome in children with severe traumatic brain injury. J. Neurotrauma. 2008;25(3):225–234. doi: 10.1089/neu.2007.0405. [DOI] [PubMed] [Google Scholar]
- 43.Lindvall O., Ernfors P., Bengzon J., Kokaia Z., Smith M.-L., Siesjö B., Persson H. Differential regulation of mRNAs for nerve growth factor, brain-derived neurotrophic factor, and neurotrophin 3 in the adult rat brain following cerebral ischemia and hypoglycemic coma. Proc. Natl. Acad. Sci. Unit. States Am. 1992;89(2):648–652. doi: 10.1073/pnas.89.2.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Branchi I., D’Andrea I., Fiore M., Di Fausto V., Aloe L., Alleva E. Early social enrichment shapes social behavior and nerve growth factor and brain-derived neurotrophic factor levels in the adult mouse brain. Biol. Psychiatr. 2006;60(7):690–696. doi: 10.1016/j.biopsych.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 45.Cekic M., Johnson S.J., Bhatt V.H., Stein D.G. Progesterone treatment alters neurotrophin/proneurotrophin balance and receptor expression in rats with traumatic brain injury. Restor. Neurol. Neurosci. 2012;30(2):115–126. doi: 10.3233/RNN-2011-0628. [DOI] [PubMed] [Google Scholar]
- 46.Bjorling D., Beckman M., Clayton M., Wang Z.-Y. Modulation of nerve growth factor in peripheral organs by estrogen and progesterone. Neuroscience. 2002;110(1):155–167. doi: 10.1016/s0306-4522(01)00568-1. [DOI] [PubMed] [Google Scholar]
- 47.Grundy P.L., Patel N., Harbuz M.S., Lightman S.L., Sharples P.M. Glucocorticoids modulate the NGF mRNA response in the rat hippocampus after traumatic brain injury. Brain Res. 2001;892(2):386–390. doi: 10.1016/s0006-8993(00)03258-3. [DOI] [PubMed] [Google Scholar]
- 48.Hamm R.J., Dixon C.E., Gbadebo D.M., Singha A.K., Jenkins L.W., Lyeth B.G., Hayes R.L. Cognitive deficits following traumatic brain injury produced by controlled cortical impact. J. Neurotrauma. 1992;9(1):11–20. doi: 10.1089/neu.1992.9.11. [DOI] [PubMed] [Google Scholar]
- 49.Yan P-s, Tang S., Zhang H-f, Guo Y-y, Zeng Z-w, Wen Q. Nerve growth factor protects against palmitic acid-induced injury in retinal ganglion cells. Neural Regen. Res. 2016;11(11):1851. doi: 10.4103/1673-5374.194758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bimonte-Nelson H.A., Nelson M.E., Granholm A.-C.E. Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain. Neuroreport. 2004;15(17):2659–2663. doi: 10.1097/00001756-200412030-00021. [DOI] [PubMed] [Google Scholar]
- 51.Young J., Pionk T., Hiatt I., Geeck K., Smith J.S. Environmental enrichment aides in functional recovery following unilateral controlled cortical impact of the forelimb sensorimotor area however intranasal administration of nerve growth factor does not. Brain Res. Bull. 2015;115:17–22. doi: 10.1016/j.brainresbull.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 52.Kamm K., VanderKolk W., Lawrence C., Jonker M., Davis A.T. The effect of traumatic brain injury upon the concentration and expression of interleukin-1β and interleukin-10 in the rat. J. Trauma Acute Care Surg. 2006;60(1):152–157. doi: 10.1097/01.ta.0000196345.81169.a1. [DOI] [PubMed] [Google Scholar]
- 53.Tarkowski E., Rosengren L., Blomstrand C., Wikkelsö C., Jensen C., Ekholm S., Tarkowski A. Early intrathecal production of interleukin-6 predicts the size of brain lesion in stroke. Stroke. 1995;26(8):1393–1398. doi: 10.1161/01.str.26.8.1393. [DOI] [PubMed] [Google Scholar]
- 54.Holmin S., Schalling M., Höjeberg B., Nordqvist A.-C.S., Skeftruna A.-K., Mathiesen T. Delayed cytokine expression in rat brain following experimental contusion. J. Neurosurg. 1997;86(3):493–504. doi: 10.3171/jns.1997.86.3.0493. [DOI] [PubMed] [Google Scholar]
- 55.Chen G., Shi J.-X., Qi M., Wang H.-X., Hang C.-H. Effects of progesterone on intestinal inflammatory response, mucosa structure alterations, and apoptosis following traumatic brain injury in male rats. J. Surg. Res. 2008;147(1):92–98. doi: 10.1016/j.jss.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 56.Raheja A., Sinha S., Samson N., Bhoi S., Subramanian A., Sharma P., Sharma B.S. Serum biomarkers as predictors of long-term outcome in severe traumatic brain injury: analysis from a randomized placebo-controlled Phase II clinical trial. J. Neurosurg. 2016;125(3):631–641. doi: 10.3171/2015.6.JNS15674. [DOI] [PubMed] [Google Scholar]
- 57.Lenzlinger P.M., Morganti-Kossmann M.-C., Laurer H.L., McIntosh T.K. The duality of the inflammatory response to traumatic brain injury. Mol. Neurobiol. 2001;24(1-3):169–181. doi: 10.1385/MN:24:1-3:169. [DOI] [PubMed] [Google Scholar]
- 58.Soltani Z., Khaksari M., Shahrokhi N., Mohammadi G., Mofid B., Vaziri A., Amiresmaili S. Effect of estrogen and/or progesterone administration on traumatic brain injury-caused brain edema: the changes of aquaporin-4 and interleukin-6. J. Physiol. Biochem. 2016;72(1):33–44. doi: 10.1007/s13105-015-0453-5. [DOI] [PubMed] [Google Scholar]
- 59.Karve I.P., Taylor J.M., Crack P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016;173(4):692–702. doi: 10.1111/bph.13125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dinarello C.A., Cannon J.G., Mancilla J., Bishai I., Lees J., Coceani F. Interleukin-6 as an endogenous pyrogen: induction of prostaglandin E2 in brain but not in peripheral blood mononuclear cells. Brain Res. 1991;562(2):199–206. doi: 10.1016/0006-8993(91)90622-3. [DOI] [PubMed] [Google Scholar]
- 61.Li X-z, Bai L-m, Yang Y-p, Luo W-f, Hu W-d, Chen J-p, Mao C-j, Liu C-f. Effects of IL-6 secreted from astrocytes on the survival of dopaminergic neurons in lipopolysaccharide-induced inflammation. Neurosci. Res. 2009;65(3):252–258. doi: 10.1016/j.neures.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Gotkin J.L., Celver J., McNutt P., Shields A.D., Howard B.C., Paonessa D.J., Napolitano P.G. Progesterone reduces lipopolysaccharide induced interleukin-6 secretion in fetoplacental chorionic arteries, fractionated cord blood, and maternal mononuclear cells. Am. J. Obstet. Gynecol. 2006;195(4):1015–1019. doi: 10.1016/j.ajog.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Dehghan F., Hadad M.K., Asadikram G., Najafipour H., Shahrokhi N. Effect of melatonin on intracranial pressure and brain edema following traumatic brain injury: role of oxidative stresses. Arch. Med. Res. 2013;44(4):251–258. doi: 10.1016/j.arcmed.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Asl S.Z., Khaksari M., Khachki A.S., Shahrokhi N., Nourizade S. Contribution of estrogen receptors alpha and beta in the brain response to traumatic brain injury. J. Neurosurg. 2013;119(2):353–361. doi: 10.3171/2013.4.JNS121636. [DOI] [PubMed] [Google Scholar]
- 65.Howell A., Osborne C.K., Morris C., Wakeling A.E. ICI 182,780 (Faslodex™) development of a novel,“pure” antiestrogen. Cancer: Interdiscipl. Int. J. Am. Cancer Soc. 2000;89(4):817–825. doi: 10.1002/1097-0142(20000815)89:4<817::aid-cncr14>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]