Figure 1.
Schematic of mucus immobilization strategy
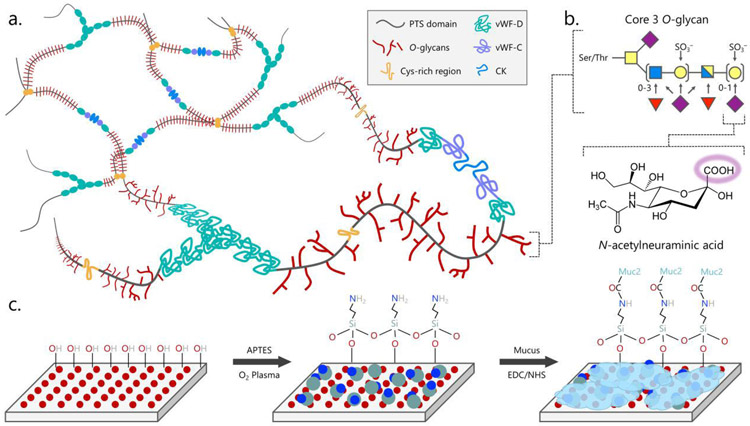
The structure of mucus presents an opportunity for covalent immobilization chemistry. Gel-forming mucins such as Muc2 form (a.) macromolecular sheets of trimers of dimers that crosslink or interact to form a mesh between von Willebrand Factor (vWF) domains and cysteine knots (CK). Each mucin monomer has a highly glycosylated proline-threonine/serine (PTS) domain with the (b.) highly sialylated core 3 O-glycan dominating in the intestines. Sialic acid, or N-acetylneuraminic acid (purple diamond), provides a freely available carboxylic acid that can be (c.) coupled to a primary amine presented on a glass or plastic surface using EDC/NHS crosslinking chemistry. Other common glycans include N-acetylglucosamine (blue square), N-acetylgalactosamine (yellow square), galactose (yellow circle), and fucose (red triangle).
*Final optimized conditions for full mucus coverage require 1% PIM, 10x higher than other conditions, and ELISA luminescence values increased proportionally.